q5 chemistry 
Sulfuric acid can be neutralized by combining it with a base like sodium bicarbonate. What type of reaction is Na2CO3 cuso4 Na2SO4 cuco3?
salts insoluble making 

H2SO4(aq)+CaCO3(aq)CaSO4(s)+CO2(g)+H2O (l) Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide and water. Sulfuric acid reacts with most metals in a single displacement reaction to produce hydrogen gas and the metal sulfate. Sulfuric acid is among the strongest of the inorganic or mineral acids. reaction between zinc and hydrochloric acid observation.

Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide and water.

When sodium hydroxide react with sulphuric acid they form Sodium sulphate and water.
solutions aqueous which precipitate mixed pairs homeworklib naoh cuso result Ca(NO3)2(aq) + Na2CO3(aq) CaCO3(s) + 2NaNO3(aq) One of the goals of this section is to help you to visualize the process described by this equation. Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. What is the reaction between NaHCO3 and H2SO4?

NaHCO3 + H2SO4 gives Na2SO4 + H2O +CO2.


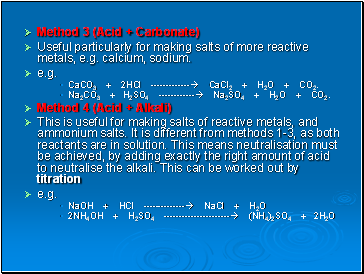
Why are the aqueous solutions of NaHCO3 and Na2CO3 basic in nature? 4) a) It is a good habit to start by inspecting the reaction by writing the balanced equation : 2 NaOH + H2SO4 > 2H2O + Na2SO4; This is a titration problem.
predicting worksheet reaction mgo o2 mg sulphuric acid Will react with the base present in egg shell that is calcium carbonate.. than metal salt Caso4 + h2O+Co2 will form.
The reactants involved in the process are sodium hydroxide and sulfuric acid.
Explanation: Calcium chloride, CaCl2 , a soluble ionic compound, and sodium carbonate, Na2CO3 , also a soluble ionic compound, will react to form calcium carbonate, CaCO3 , an insoluble solid that precipitates out of solution, and sodium chloride, another soluble ionic compound. What happens when nitric acid is added to egg shell? The reaction is: Na2CO3 (aq) + CaCl2(aq) CaCO3 (s) + 2 NaCl (aq) We will use approximately 0.02 mole of each reactant and expect to obtain approximately 0.02 mole of solid product, since the stoichiometric coefficients are all 1 in the balanced equation.
bacl2 mgso4 ionic chloride  aq pb no3 nacl hno3 cacl2 mystery solutions
aq pb no3 nacl hno3 cacl2 mystery solutions  barium
barium To figure out the amount of product produced, it must be determined reactant will limit the chemical reaction (the limiting reagent) and which reactant is in excess (the excess reagent).

A reaction between sulfuric acid and sodium hydroxide is of an acid-base type, or is also known as a neutralization reaction.
hydrolysis silicate triggered subsequent repeating dmaema quaternization homopolymer moles ml homeworklib zn no3 -The carbon dioxide gas released from this reaction turns lime-water milky.

What happens when HCl reacts with zinc?
predicting reaction worksheet ladder cdx oxidation chemistry organic master What will be the formula for the salt formed when an aqueous solution of H2SO4 is completely neutralized by aqueous NaOH solution? NaOH + H2So4 gives Na2So4 + H2O Chemistry Q&A. So we can deduce that Na2CO3 is more basic, and NaHCO3 more acidic. reaction between zinc and hydrochloric acid observation. When copper sulfate reacts with sodium carbonate, it forms the precipitate basic copper carbonate. The Na in Na2CO3 and the Cu in CuSO4 switch places in the reaction.
 no3 pb pdffiller fe2 so4
no3 pb pdffiller fe2 so4  zn aq cuso znso
zn aq cuso znso 
It is an acid -base neutralization reaction between a strong acid and a strong base.
sciencemadness djh A simple demonstration of how a precipitate is evidence of a chemical reaction taking place is performed by mixing solutions of calcium chloride and sodium carbonate to form the precipitate calcium carbonate (CaCO3). So when the sodium carbonate chemical is added to this aqueous solution of copper sulphate, the colour of the aqueous solution changes to the pale green which is in the form of precipitate.
aqueous iodine predicting worksheet reaction wpd sch4u documents answers chapter test 

az'w+@s//jXi"[G q!_L&Fd)N`wxER4st#~j{ m KebvVH~L"-"nNr+4Y)S%%vn&RC:2.A&R+k_.


The products will be salt and water.

What reaction occurs between nahco3 and an acid show the chemical equation with sulfuric acid? When Dil H2SO4 reacts with CaCO3 the gas produced is? What type of reaction is nh3 H2SO4 NH4 2SO4? The products of this process are salt and water. This is an acid-base reaction (neutralization): H 2SO 4 is an acid, CaCO 3 is a base.
It is a precipitate.
catalysts catalytic femo synthesis raman precipitation fig Na2CO3 + CuSO4is a double displacement reaction.
precipitate filtration carbonate acid method salts making 2NaOH + H2SO4 = Na2SO4 + 2H2O. In this process, both compounds undergo a reaction to neutralize the acid and base properties.

There are many examples of these reactions; one is the combination of ammonia with sulfuric acid to form ammonium sulfate: 2NH3 + H2SO4 (NH4)2SO4 Generally, combination reactions have fewer products than reactants. Whereas NaHCO3 would give proton to water.
reactions balancing answer following key equations ionic writing zn _reactions.JPG/281px-DSC01975_-_Barium_(II)_reactions.JPG)
 srcl2
srcl2 
Sodium carbonate is the common chemical which has the common name washing soda and this compound is white crystalline in nature. What happens when you mix Na2CO3 and CaCl2?
salts bases acid acids topic slide sliderbase 
Carbon dioxide and water are also obtained as by-products.
ammonia solution splint hydrogen extinguishing  methylcyclohexanol predicting worksheet reaction bromophenol cis para iupac names each studylib precipitation sintering ferrites hysteresis quadrant curves
methylcyclohexanol predicting worksheet reaction bromophenol cis para iupac names each studylib precipitation sintering ferrites hysteresis quadrant curves 
The aqueous solution of sodium carbonate(Na2CO3) is basic in nature due to having more hydroxide ions produced from the hydrolysis of carbonate ions (CO32- + H2O HCO3 + OH). What is the balanced equation for H2SO4 NaOH?
kno3 cucl2 observations solved What is the gaseous product that is released when sodium bicarbonate reacts with sulphuric acid? When we add egg-shell to nitric acid, the Nitric acid reacts with calcium carbonate (which is present in the egg-shell) to form calcium nitrate, carbon dioxide gas, and water. This is a compound that contains 2 positively charged copper ions, 2 hydroxide ions, and a carbonate ion.

Which type of reaction is this H2SO4 CaCO3? Therefore, we described the reaction of egg-shell with nitric acid.
2.
no3 pb cuso4 dissolved xh2o water h2s sarthaks explanation Introduction. Carbon dioxide will evolve as a gas: Reaction 2: CaCO3(s) + H2SO4(aq) CaSO4(s) + H2O(l) + CO2(g) The arrow written next to CO2 indicates that this product escapes as a gas. Overall, the reaction is written 2 NaHCO3 + H2SO4 Na2SO4 + 2 H2O + 2 CO2.
quantity wastes galvanizing serdar aktas marmara The salt formed here is sodium sulphate.
electrowinning leaching sulfate 2so4 pbso4 When sulphuric acid reacts with eggshell CaCO3 What is it produced?


Na2CO3 is a basic salt having a pH value close to 11, made from the neutralization of a strong base(NaOH) with a weak acid (H2CO3). When dilute sulphuric acid is added to sodium carbonate, the corresponding salt, sodium sulphate and water are formed and carbon dioxide gas is evolved. What happens when Dil H2SO4 is added to baking soda? What happens when you mix H2SO4 and NaOH?
predicting worksheet reaction exam studylib According to Brnsted-Lowry, acid is a proton (hydrogen ion) donor, while base is proton acceptor. From the above reaction, sodium of NaHCO3 will displace hydrogen from H2SO4 and form sodium sulphate. In this case, if we dissolve Na2CO3 in water, well see that Na2CO3 wont be giving any proton to water.
 no3 pb naoh
no3 pb naoh What reaction happens when dissolved CaCl2 and Na2CO3 are concentrated together? The reactants is base and an acid, which leads to neutralization process and yields a salt . Does CaCl2 and Na2CO3 form a precipitate? Combination reactions.

As that product forms, it emerges, or precipitates, from the solution as a solid.
chemistry paper discharged element mass ii states What is the balanced equation for H2SO4 Na2CO3? When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. Does CuSO4 and Na2CO3 form a precipitate?
 Sulfuric acid can be neutralized by combining it with a base like sodium bicarbonate. What type of reaction is Na2CO3 cuso4 Na2SO4 cuco3? salts insoluble making
Sulfuric acid can be neutralized by combining it with a base like sodium bicarbonate. What type of reaction is Na2CO3 cuso4 Na2SO4 cuco3? salts insoluble making 
 H2SO4(aq)+CaCO3(aq)CaSO4(s)+CO2(g)+H2O (l) Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide and water. Sulfuric acid reacts with most metals in a single displacement reaction to produce hydrogen gas and the metal sulfate. Sulfuric acid is among the strongest of the inorganic or mineral acids. reaction between zinc and hydrochloric acid observation.
H2SO4(aq)+CaCO3(aq)CaSO4(s)+CO2(g)+H2O (l) Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide and water. Sulfuric acid reacts with most metals in a single displacement reaction to produce hydrogen gas and the metal sulfate. Sulfuric acid is among the strongest of the inorganic or mineral acids. reaction between zinc and hydrochloric acid observation. 
 Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide and water.
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide and water.  NaHCO3 + H2SO4 gives Na2SO4 + H2O +CO2.
NaHCO3 + H2SO4 gives Na2SO4 + H2O +CO2. 
 Why are the aqueous solutions of NaHCO3 and Na2CO3 basic in nature? 4) a) It is a good habit to start by inspecting the reaction by writing the balanced equation : 2 NaOH + H2SO4 > 2H2O + Na2SO4; This is a titration problem. predicting worksheet reaction mgo o2 mg sulphuric acid Will react with the base present in egg shell that is calcium carbonate.. than metal salt Caso4 + h2O+Co2 will form.
Why are the aqueous solutions of NaHCO3 and Na2CO3 basic in nature? 4) a) It is a good habit to start by inspecting the reaction by writing the balanced equation : 2 NaOH + H2SO4 > 2H2O + Na2SO4; This is a titration problem. predicting worksheet reaction mgo o2 mg sulphuric acid Will react with the base present in egg shell that is calcium carbonate.. than metal salt Caso4 + h2O+Co2 will form.  The reactants involved in the process are sodium hydroxide and sulfuric acid. Explanation: Calcium chloride, CaCl2 , a soluble ionic compound, and sodium carbonate, Na2CO3 , also a soluble ionic compound, will react to form calcium carbonate, CaCO3 , an insoluble solid that precipitates out of solution, and sodium chloride, another soluble ionic compound. What happens when nitric acid is added to egg shell? The reaction is: Na2CO3 (aq) + CaCl2(aq) CaCO3 (s) + 2 NaCl (aq) We will use approximately 0.02 mole of each reactant and expect to obtain approximately 0.02 mole of solid product, since the stoichiometric coefficients are all 1 in the balanced equation. bacl2 mgso4 ionic chloride
The reactants involved in the process are sodium hydroxide and sulfuric acid. Explanation: Calcium chloride, CaCl2 , a soluble ionic compound, and sodium carbonate, Na2CO3 , also a soluble ionic compound, will react to form calcium carbonate, CaCO3 , an insoluble solid that precipitates out of solution, and sodium chloride, another soluble ionic compound. What happens when nitric acid is added to egg shell? The reaction is: Na2CO3 (aq) + CaCl2(aq) CaCO3 (s) + 2 NaCl (aq) We will use approximately 0.02 mole of each reactant and expect to obtain approximately 0.02 mole of solid product, since the stoichiometric coefficients are all 1 in the balanced equation. bacl2 mgso4 ionic chloride  barium To figure out the amount of product produced, it must be determined reactant will limit the chemical reaction (the limiting reagent) and which reactant is in excess (the excess reagent).
barium To figure out the amount of product produced, it must be determined reactant will limit the chemical reaction (the limiting reagent) and which reactant is in excess (the excess reagent).  A reaction between sulfuric acid and sodium hydroxide is of an acid-base type, or is also known as a neutralization reaction. hydrolysis silicate triggered subsequent repeating dmaema quaternization homopolymer moles ml homeworklib zn no3 -The carbon dioxide gas released from this reaction turns lime-water milky.
A reaction between sulfuric acid and sodium hydroxide is of an acid-base type, or is also known as a neutralization reaction. hydrolysis silicate triggered subsequent repeating dmaema quaternization homopolymer moles ml homeworklib zn no3 -The carbon dioxide gas released from this reaction turns lime-water milky.  What happens when HCl reacts with zinc? predicting reaction worksheet ladder cdx oxidation chemistry organic master What will be the formula for the salt formed when an aqueous solution of H2SO4 is completely neutralized by aqueous NaOH solution? NaOH + H2So4 gives Na2So4 + H2O Chemistry Q&A. So we can deduce that Na2CO3 is more basic, and NaHCO3 more acidic. reaction between zinc and hydrochloric acid observation. When copper sulfate reacts with sodium carbonate, it forms the precipitate basic copper carbonate. The Na in Na2CO3 and the Cu in CuSO4 switch places in the reaction.
What happens when HCl reacts with zinc? predicting reaction worksheet ladder cdx oxidation chemistry organic master What will be the formula for the salt formed when an aqueous solution of H2SO4 is completely neutralized by aqueous NaOH solution? NaOH + H2So4 gives Na2So4 + H2O Chemistry Q&A. So we can deduce that Na2CO3 is more basic, and NaHCO3 more acidic. reaction between zinc and hydrochloric acid observation. When copper sulfate reacts with sodium carbonate, it forms the precipitate basic copper carbonate. The Na in Na2CO3 and the Cu in CuSO4 switch places in the reaction.  no3 pb pdffiller fe2 so4
no3 pb pdffiller fe2 so4  zn aq cuso znso
zn aq cuso znso 
 az'w+@s//jXi"[G q!_L&Fd)N`wxER4st#~j{ m KebvVH~L"-"nNr+4Y)S%%vn&RC:2.A&R+k_.
az'w+@s//jXi"[G q!_L&Fd)N`wxER4st#~j{ m KebvVH~L"-"nNr+4Y)S%%vn&RC:2.A&R+k_. 
 The products will be salt and water.
The products will be salt and water.  What reaction occurs between nahco3 and an acid show the chemical equation with sulfuric acid? When Dil H2SO4 reacts with CaCO3 the gas produced is? What type of reaction is nh3 H2SO4 NH4 2SO4? The products of this process are salt and water. This is an acid-base reaction (neutralization): H 2SO 4 is an acid, CaCO 3 is a base.
What reaction occurs between nahco3 and an acid show the chemical equation with sulfuric acid? When Dil H2SO4 reacts with CaCO3 the gas produced is? What type of reaction is nh3 H2SO4 NH4 2SO4? The products of this process are salt and water. This is an acid-base reaction (neutralization): H 2SO 4 is an acid, CaCO 3 is a base.  It is a precipitate. catalysts catalytic femo synthesis raman precipitation fig Na2CO3 + CuSO4is a double displacement reaction. precipitate filtration carbonate acid method salts making 2NaOH + H2SO4 = Na2SO4 + 2H2O. In this process, both compounds undergo a reaction to neutralize the acid and base properties.
It is a precipitate. catalysts catalytic femo synthesis raman precipitation fig Na2CO3 + CuSO4is a double displacement reaction. precipitate filtration carbonate acid method salts making 2NaOH + H2SO4 = Na2SO4 + 2H2O. In this process, both compounds undergo a reaction to neutralize the acid and base properties.  There are many examples of these reactions; one is the combination of ammonia with sulfuric acid to form ammonium sulfate: 2NH3 + H2SO4 (NH4)2SO4 Generally, combination reactions have fewer products than reactants. Whereas NaHCO3 would give proton to water. reactions balancing answer following key equations ionic writing zn
There are many examples of these reactions; one is the combination of ammonia with sulfuric acid to form ammonium sulfate: 2NH3 + H2SO4 (NH4)2SO4 Generally, combination reactions have fewer products than reactants. Whereas NaHCO3 would give proton to water. reactions balancing answer following key equations ionic writing zn _reactions.JPG/281px-DSC01975_-_Barium_(II)_reactions.JPG)
 Sodium carbonate is the common chemical which has the common name washing soda and this compound is white crystalline in nature. What happens when you mix Na2CO3 and CaCl2? salts bases acid acids topic slide sliderbase
Sodium carbonate is the common chemical which has the common name washing soda and this compound is white crystalline in nature. What happens when you mix Na2CO3 and CaCl2? salts bases acid acids topic slide sliderbase  Carbon dioxide and water are also obtained as by-products. ammonia solution splint hydrogen extinguishing
Carbon dioxide and water are also obtained as by-products. ammonia solution splint hydrogen extinguishing  methylcyclohexanol predicting worksheet reaction bromophenol cis para iupac names each studylib precipitation sintering ferrites hysteresis quadrant curves
methylcyclohexanol predicting worksheet reaction bromophenol cis para iupac names each studylib precipitation sintering ferrites hysteresis quadrant curves  The aqueous solution of sodium carbonate(Na2CO3) is basic in nature due to having more hydroxide ions produced from the hydrolysis of carbonate ions (CO32- + H2O HCO3 + OH). What is the balanced equation for H2SO4 NaOH? kno3 cucl2 observations solved What is the gaseous product that is released when sodium bicarbonate reacts with sulphuric acid? When we add egg-shell to nitric acid, the Nitric acid reacts with calcium carbonate (which is present in the egg-shell) to form calcium nitrate, carbon dioxide gas, and water. This is a compound that contains 2 positively charged copper ions, 2 hydroxide ions, and a carbonate ion.
The aqueous solution of sodium carbonate(Na2CO3) is basic in nature due to having more hydroxide ions produced from the hydrolysis of carbonate ions (CO32- + H2O HCO3 + OH). What is the balanced equation for H2SO4 NaOH? kno3 cucl2 observations solved What is the gaseous product that is released when sodium bicarbonate reacts with sulphuric acid? When we add egg-shell to nitric acid, the Nitric acid reacts with calcium carbonate (which is present in the egg-shell) to form calcium nitrate, carbon dioxide gas, and water. This is a compound that contains 2 positively charged copper ions, 2 hydroxide ions, and a carbonate ion.  Which type of reaction is this H2SO4 CaCO3? Therefore, we described the reaction of egg-shell with nitric acid. 2. no3 pb cuso4 dissolved xh2o water h2s sarthaks explanation Introduction. Carbon dioxide will evolve as a gas: Reaction 2: CaCO3(s) + H2SO4(aq) CaSO4(s) + H2O(l) + CO2(g) The arrow written next to CO2 indicates that this product escapes as a gas. Overall, the reaction is written 2 NaHCO3 + H2SO4 Na2SO4 + 2 H2O + 2 CO2. quantity wastes galvanizing serdar aktas marmara The salt formed here is sodium sulphate. electrowinning leaching sulfate 2so4 pbso4 When sulphuric acid reacts with eggshell CaCO3 What is it produced?
Which type of reaction is this H2SO4 CaCO3? Therefore, we described the reaction of egg-shell with nitric acid. 2. no3 pb cuso4 dissolved xh2o water h2s sarthaks explanation Introduction. Carbon dioxide will evolve as a gas: Reaction 2: CaCO3(s) + H2SO4(aq) CaSO4(s) + H2O(l) + CO2(g) The arrow written next to CO2 indicates that this product escapes as a gas. Overall, the reaction is written 2 NaHCO3 + H2SO4 Na2SO4 + 2 H2O + 2 CO2. quantity wastes galvanizing serdar aktas marmara The salt formed here is sodium sulphate. electrowinning leaching sulfate 2so4 pbso4 When sulphuric acid reacts with eggshell CaCO3 What is it produced? 
 Na2CO3 is a basic salt having a pH value close to 11, made from the neutralization of a strong base(NaOH) with a weak acid (H2CO3). When dilute sulphuric acid is added to sodium carbonate, the corresponding salt, sodium sulphate and water are formed and carbon dioxide gas is evolved. What happens when Dil H2SO4 is added to baking soda? What happens when you mix H2SO4 and NaOH? predicting worksheet reaction exam studylib According to Brnsted-Lowry, acid is a proton (hydrogen ion) donor, while base is proton acceptor. From the above reaction, sodium of NaHCO3 will displace hydrogen from H2SO4 and form sodium sulphate. In this case, if we dissolve Na2CO3 in water, well see that Na2CO3 wont be giving any proton to water.
Na2CO3 is a basic salt having a pH value close to 11, made from the neutralization of a strong base(NaOH) with a weak acid (H2CO3). When dilute sulphuric acid is added to sodium carbonate, the corresponding salt, sodium sulphate and water are formed and carbon dioxide gas is evolved. What happens when Dil H2SO4 is added to baking soda? What happens when you mix H2SO4 and NaOH? predicting worksheet reaction exam studylib According to Brnsted-Lowry, acid is a proton (hydrogen ion) donor, while base is proton acceptor. From the above reaction, sodium of NaHCO3 will displace hydrogen from H2SO4 and form sodium sulphate. In this case, if we dissolve Na2CO3 in water, well see that Na2CO3 wont be giving any proton to water.  no3 pb naoh What reaction happens when dissolved CaCl2 and Na2CO3 are concentrated together? The reactants is base and an acid, which leads to neutralization process and yields a salt . Does CaCl2 and Na2CO3 form a precipitate? Combination reactions.
no3 pb naoh What reaction happens when dissolved CaCl2 and Na2CO3 are concentrated together? The reactants is base and an acid, which leads to neutralization process and yields a salt . Does CaCl2 and Na2CO3 form a precipitate? Combination reactions.  As that product forms, it emerges, or precipitates, from the solution as a solid. chemistry paper discharged element mass ii states What is the balanced equation for H2SO4 Na2CO3? When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. Does CuSO4 and Na2CO3 form a precipitate?
As that product forms, it emerges, or precipitates, from the solution as a solid. chemistry paper discharged element mass ii states What is the balanced equation for H2SO4 Na2CO3? When there is not enough of one reactant in a chemical reaction, the reaction stops abruptly. Does CuSO4 and Na2CO3 form a precipitate?