The water molecules stay bonded to each other and the non-polar molecules can't dissolve. For example, if hydrophobicity is too dominant, then an amorphous cluster of residues with few native contacts can be formed rather than a correctly folded protein [19]. In addition, the comparison of the value of the normalisation constant (0.94 ) with the values of the and terms (0.49 and 1.24 , respectively) in the HP-HB decomposition of the SJKG matrix confirms that the value of does not affect the ratio of for native proteins and that this ratio is reversed between folded globular proteins and amyloid fibrils. At the higher temperature, when water molecules become more mobile, this energy gain decreases along with the entropic component. This simple decomposition given by Eq. (B) Parallel matrix, , rmsd 0.42 . 1A) have all revealed that residue-water interactions play a dominant role in protein folding [6][8]. Chang, Raymond. Result: \( \Delta{G} \) is negative and hence hydrophobic interactions are spontaneous. 1D,E,F) it is clear that the free energy of forming hydrophobic-polar (H-P) side-chain contacts is stabilising for globular proteins although not nearly as important in the simple formation of -sheets. These weightings are in excellent agreement with a modified form of the HP model [29] ( in the present study compared to 2.3 in the modified HP model [29]) and so validate its use in protein folding simulations. For protein misfolding and amyloid formation, the ratio of to for both PASTA matrices is (Table 1) suggesting that backbone-only hydrogen bonding is about 50% more important in stabilising amyloid fibrils than hydrophobic interactions. The pH of a solution is a measure of the concentration of hydrogen ions in the solution. Similar experiments for protein aggregation, however, reveal that peptides with removed backbone amide groups have a much reduced propensity to form ordered aggregates [50]; indeed such species are being explored as potential therapeutic inhibitors of amyloid fibril growth [51]. Overall, however, this balance appears to be very finely tuned for both protein folding and misfolding, and it is interesting to speculate on the role of this delicate balance of forces within the cell. We decomposed the MJ and PASTA matrices into a combination of the HP (Hydrophobic-Polar) model [11] and a backbone hydrogen bonding model in which all amino acids, except for proline, are capable of forming backbone hydrogen bonds (by analogy, we term this the HB model).
website. Minimizing the number of hydrophobic side chains exposed to water is the principal driving force behind the folding process,[8][9][10] although formation of hydrogen bonds within the protein also stabilizes protein structure. This expression, therefore, describes the original data extremely well and suggests that the diverse and complex interactions stabilising both the native and fibrillar states are amenable to a low-dimensional representation using simple two-body and one-body terms [6][8]. For random heteropolymers, the pairwise contact free energies can be approximated as a set of 210 independent random variables (i.e. These cohesive forces are also related to the waters property of adhesion, or the attraction between water molecules and other molecules. This means that water moderates temperature changes within organisms and in their environments.

A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. Water molecules that are distorted by the presence of the hydrophobe will make new hydrogen bonds and form an ice-like cage structure called a clathrate cage around the hydrophobe. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. If you're seeing this message, it means we're having trouble loading external resources on our website. This assumption is supported by the observation that the PASTA matrices are highly successful at predicting the portions of a polypeptide sequence that stabilise the core regions of experimentally determined amyloid fibrils and the intra-sheet registry of the -sheets [9]. No, Is the Subject Area "Hydrogen bonding" applicable to this article?
hydrophilic molecules philic organic acetic hydro acid common chemistry chem igoc ucla harding edu 2A,B,C). Such a shift in balance can be seen as a jump between free energy landscape minima, and could occur, for example, at a critical concentration [58], or pH [55], or at a temperature sufficiently high to overcome kinetic barriers between the native and amyloid minima [46]. The inclusion of the HP term in Eq. Hydrophobic Interactions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Hence the hydrophobic effect is essential to life. Northern Arizona University: Polar and Non-Polar Molecules. Nevertheless, hydrogen bonding does play an important role in protein folding since highly polar sequences can fold to form -helices, and side-chain only molecular dynamics simulations fail to capture crucial aspects of protein folding [24]. These results characterise the nature of the interactions that determine the competition between folding and misfolding of proteins by revealing that the stability of native proteins is primarily determined by hydrophobic interactions between side-chains, while the stability of amyloid fibrils depends more on backbone intermolecular hydrogen bonding interactions. Have you ever filled up a glass of water to the very top and then slowly added a few more drops? [21], which we refer to as the SJKG matrix, explicitly includes effects due to chain connectivity.
In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. When existing parallel and antiparallel -sheet propensity scales [31] are converted into free energies (Table 2, column 5 and 6 respectively), grouped into polar and non-polar terms and then separately shifted to have zero mean, thus removing the average hydrophobic (hydrophilic) cost (gain) to water of forming a sheet (the values are +0.32 (0.51 ) and +0.34 (0.25 ) for parallel and antiparallel -sheets respectively), the remainder correlates extremely well with (correlation coefficients of 0.96 and 0.97 for parallel and antiparallel -sheets respectively, Fig. These two-body interactions are described by three interaction matrices, , and , with the following properties: if and are both hydrophobic residues and topological neighbours, and otherwise; if either or is a hydrophobic residue, and are topological neighbours, and otherwise; if and can both form backbone hydrogen bonds and are topological neighbours, otherwise . Non-polar molecules don't have differently charged ends and so can't break the hydrogen bonds of the water molecules. The H+ ions can combine with the OH ions, limiting the increase in pH. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Physical Chemistry for the Life Sciences. The water molecules that form the "cage" (or clathrate) have restricted mobility. Firstly, the value of is only slightly affected by considering amino acids such as Proline and Alanine to be hydrophilic rather than hydrophobic. Increased energy disrupts the hydrogen bonds between water molecules. [1] but have different amino acid interaction matrices , according to Eq. Sausalito, CA: Edwards Brothers, Inc. 2005. [6] describing protein folding and misfolding is analogous to that of a spin glass model in which competition between conflicting interactions leads to a rugged free energy landscape [41]. American chemist Walter Kauzmann discovered that nonpolar substances like fat molecules tend to clump up together rather than distributing itself in a water medium, because this allow the fat molecules to have minimal contact with water. Since the Hamiltonian maps the sequence space on to the structure space, as the weights , and change so too does the shape of the resulting structure. This situation occurs as the competing processes of protein folding and misfolding are finely tuned in terms of their free energies. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.11). The twenty one-body terms, , were determined by performing a simulated annealing minimisation in MATLAB. This approach suggests that while hydrophobicity, hydrogen bonding and the formation of secondary structure are important to both processes, the balance between hydrophobicity and hydrogen bonding is remarkably different in the two regimes. Column chromatography with a hydrophobic stationary phase such as phenyl-sepharose will cause more hydrophobic proteins to travel more slowly, while less hydrophobic ones elute from the column sooner. One of the most studied matrices of this type has been reported by Miyazawa and Jernigan [5]. 2022 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved.
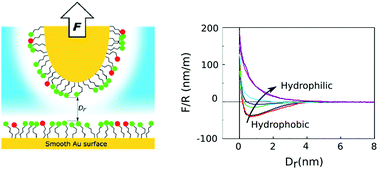
We also note that in the -sheets of globular proteins, the effects of backbone hydrogen bonding tends to be averaged out in Eq. Waters cohesive forces allow for the property of surface tension. This is because the water molecules are attracted to the straw and therefore adhere to it.

Blood Flow and Blood Pressure Regulation, 22.2. https://doi.org/10.1371/journal.pcbi.1002169.t001. Previous work has shown that one-body components of the MJ matrix, known as q-values, are closely related to the interactions governing secondary structure formation [6]. These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) (Figure 2.8 a).
cellulose hydrophobic hydrophilic adapted interconversions polymorphs hypothetical synthase representing This effect is even more apparent in the simpler case of the one-body components of the parallel and antiparallel PASTA matrices (Table 2, parallel and antiparallel respectively). This interpretation implies that globular proteins are stabilised mainly by side-chain hydrophobic interactions [6] since the sum of all H-H, H-P and P-P contacts captures the overall distribution of contact free energies extremely well (Fig. This result suggests that the segregation of hydrophobic and polar residues is not very important in -sheet formation and could lead to solvent exposed non-polar side-chains in prefibrillar aggregates, a feature that has been suggested to be closely linked to cytotoxicity [30]. However, a knowledge-based pair potential for describing amino acid interactions in the native folds of globular proteins developed by Skolnick, et al. acid: a substance that donates hydrogen ions and therefore lowers pH, adhesion: the attraction between water molecules and molecules of a different substance, base: a substance that absorbs hydrogen ions and therefore raises pH, buffer: a solution that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions, cohesion: the intermolecular forces between water molecules caused by the polar nature of water; creates surface tension, evaporation: the release of water molecules from liquid water to form water vapor, hydrophilic: describes a substance that dissolves in water; water-loving, hydrophobic: describes a substance that does not dissolve in water; water-fearing, litmus paper: filter paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, pH scale: a scale ranging from 0 to 14 that measures the approximate concentration of hydrogen ions of a substance, solvent: a substance capable of dissolving another substance, surface tension: the cohesive force at the surface of a body of liquid that prevents the molecules from separating, temperature: a measure of molecular motion, Humphrey, W., Dalke, A. and Schulten, K., VMDVisual Molecular Dynamics, J. Molec. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. the 210 independent elements in a symmetric matrix). 1B,C). We also note that the MJ matrix is calculated by using the quasi-chemical approximation in which protein residues are assumed to be in equilibrium with the solvent. Hydrogen bonds are not readily formed with nonpolar substances like oils and fats . Buffers are important in biological systems because of their ability to maintain constant pH conditions. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Major advances in establishing the interactions that drive the folding process have been made by analysing the structures in the Protein Data Bank (PDB), and particularly by examining the frequency with which contacts between the different types of amino acid residues occur [5].
hydrophilic hydrophobic hydrophilic hydrophobic (E,F) Single Gaussian fits to the distributions of parallel (E) and antiparallel (F) contact free energies (0.51, s.d. Yes So how is it that we can ingest or inhale acidic or basic substances and not die?

This means that ice floats on the surface of a body of water (Figure 2.8 b). Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! All of these unique properties of water are important in the chemistry of living organisms. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems. More bonds are broken than are formed. Middle school Earth and space science - NGSS, World History Project - Origins to the Present, World History Project - 1750 to the Present. [17] In the case of larger nonpolar molecules, the reorientational and translational motion of the water molecules in the solvation shell may be restricted by a factor of two to four; thus, at 25C the reorientational correlation time of water increases from 2 to 4-8 picoseconds.
hydrophobic hydrogen phthalate vitamin parts hydrophilic methyl indicate problems practice mol problem chemistry organic molecular Interaction parameters to describe the folding process are usually defined by considering a subspace that includes the regions of conformational space corresponding to the native states of globular proteins [19]. In addition, the type of amino acid and specific sequence patterns have varying degrees of globularity [18] or aggregation propensity [16] with certain amino acids, notably proline, appearing much more frequently in globular proteins than in the core region of amyloid fibrils [9].
This means these materials, made up of non-polar molecules, are hydrophobic or water-repellent. Temperature is a measure of the motion (kinetic energy) of molecules.

1A, top left corner, blue), followed by hydrophobic-polar (Fig. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation. Thus, this result strengthens our findings as the hydrophobicity term, , is even more dominant than the hydrogen bonding term, , in the decomposition of the SJKG matrix than in the MJ matrix ( ratios of 2.50 and 2.08 respectively). Donate or volunteer today! and measure the additional free energy of forming hydrophobic contacts and the free energy gained through hydrogen bond formation. We find that our equivalent one-body potentials, MJ (Table 2), correlate extremely well with (correlation coefficient of 0.98, Fig. Failure to fold, or to remain folded correctly, may result in misfolding and aggregation, which are processes associated with a wide range of highly debilitating, and so far incurable, human conditions that include Alzheimer's and Parkinson's diseases and type II diabetes. [3] has only a marginal effect on the regression to the parallel or antiparallel matrices as demonstrated by the relatively small coefficient 0.2 (Table 1). [6]. [21] conclude that ignoring chain connectivity does not introduce errors and that the quasi-chemical approximation is sufficient for extracting statistical potentials such as the MJ matrix.
 hydrophobic hydrophilic zeolite
hydrophobic hydrophilic zeolite Water has many properties that are critical to maintaining life. here. These nonpolar compounds are hydrophobic (water-fearing) and will not dissolve in water. Oxford, UK: Oxford University Press. The water molecules form comparatively weak intermolecular bonds called hydrogen bonds. Skolnick, et al. Conversely, bases are those substances that readily donate OH.
hydrophilic hydrophobic pva emulsion Upon folding, the protein minimises the free energy of the protein-water system by clustering hydrophobic groups and forming intramolecular hydrogen bonds in the globular interior. Tearing down a portion of the clathrate cage will cause the entropy to increase ( \( \Delta S \) is positive), since forming it decreases the entropy. Legal. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A positive \(\Delta G\) indicates that the mixing of the hydrophobe and water molecules is not spontaneous. [4] into Eq. Recent experimental evidence supports this interpretation of protein folding and misfolding. The resulting parallel strand and antiparallel strand interaction free energy matrices (referred to here as parallel and antiparallel respectively) are shown in Fig. Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. However, the amino acid sequences of individual peptides and proteins influence their specific propensity to aggregate [16], [17], and to form self-complementary side-chain packing interfaces between adjacent -sheets in the fibrils [15], [27], [28]. In the solvation shell of small nonpolar particles, the restriction amounts to some 10%. This approach enables us to analyse quantitatively the relative importance of local and non-local interactions in determining the folding and misfolding of proteins. The normalisation constant shifts the elements of the MJ and PASTA matrices along the free energy axis thus allowing comparison of , and between different matrices. Furthermore, if we adopt the partitioning suggested by Li, et al. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. This orientation makes the system (hydrophobe) more structured with an decrease of the total entropy of the system; therefore \( \Delta S < 0\). Ions are hydrophilic or attracted to water molecules because the water molecules are polar, with a negative charge at one end and a positive charge at the other end. This ratio was corroborated by decomposing three recent pairwise contact potentials for the native states of globular proteins [21][23] which gave a similar result ( values are 0.4 [21], 0.7 [22], 0.73 [23] and on average).
Hence, we suggest that the one-body free energy terms, , correspond to a stabilisation of the native or fibrillar state through a competition between hydrophilicity and the formation of hydrogen bonded secondary structure. The end of the water molecule with the oxygen atom is negatively charged while the hydrogen atom end is positively charged. This generic view [12] is consistent with the observation that even hydrophilic and homopolymeric sequences of amino acids can form amyloid fibrils [13]. Preface to the original textbook, by OpenStax College, 3.2 Comparing Prokaryotic and Eukaryotic Cells, 4.3 Citric Acid Cycle and Oxidative Phosphorylation, 4.5 Connections to Other Metabolic Pathways, 5.2: The Light-Dependent Reactions of Photosynthesis, 8.3 Extensions of the Laws of Inheritance, 10.2 Biotechnology in Medicine and Agriculture, 20.2 Gas Exchange across Respiratory Surfaces, 20.4 Transport of Gases in Human Bodily Fluids, 21.4. This intricate interplay of competing interactions gives rise to multiple local minima in the effective energy function of Eq. Concepts of Biology - 1st Canadian Edition by Charles Molnar and Jane Gair is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. (2) by the presence of other secondary structure motifs (-helices, -turns and coil).
hydrophobic hydrophilic vs between hydrophile difference acids amino The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. On closer inspection, analysis of these interactions in the form of a histogram shows that the distribution of contact free energies determined from the Miyazawa-Jernigan (MJ) matrix (Fig. This procedure removes an average hydrophobic penalty for non-polar residues (+1.45 ) and an average hydrophilic gain for polar residues (0.07 ). Likewise, if too much OH is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. with a small unknown value of \(\Delta H\) and a large negative value of \(\Delta{S} \), the value of \(\Delta G\) will turn out to be positive. Since , and are all binary matrices, it is straightforward to quantify the marginal effect of each of the regressors in our general linear model from the values of their coefficients , and . By virtue of using native reference states, the SJKG matrix has both positive and negative side-chain interaction free energies and is similar in this way to the PASTA matrices (Fig. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. We find that the minima of the protein free energy landscape for folding and misfolding tend to be respectively dominated by hydrophobic and by hydrogen bonding interactions. A water molecule is formed from two hydrogen atoms and an oxygen atom linked by two polar covalent bonds. Is the Subject Area "Free energy" applicable to this article? Cells no longer function properly, and proteins will break down. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. This suggests that the monomer-monomer association energy is a linear decreasing function of denaturant concentration under mildly denaturing conditions. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Competing interests: The authors have declared that no competing interests exist. The SJKG matrix also has a mean free energy of approximately zero (0.08 ) like the PASTA matrices (0.51 and 0.13 for parallel and antiparallel respectively, Fig. Since proline cannot form inter-molecular backbone hydrogen bonds this observation suggests that the stabilisation of -sheets arises mainly from the dominance of backbone hydrogen bonding, with hydrophobic interactions (Fig. This is observed when water climbs up a straw placed in a glass of water. [13][14], In biochemistry, the hydrophobic effect can be used to separate mixtures of proteins based on their hydrophobicity. In our initial classification of hydrophobic and hydrophilic residues [11], the ratios between the hydrogen bonding and hydrophobic terms, , are 0.48, 1.59 and 1.39 for the MJ, parallel and antiparallel PASTA matrices respectively (Table 1).
 A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. Water molecules that are distorted by the presence of the hydrophobe will make new hydrogen bonds and form an ice-like cage structure called a clathrate cage around the hydrophobe. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. If you're seeing this message, it means we're having trouble loading external resources on our website. This assumption is supported by the observation that the PASTA matrices are highly successful at predicting the portions of a polypeptide sequence that stabilise the core regions of experimentally determined amyloid fibrils and the intra-sheet registry of the -sheets [9]. No, Is the Subject Area "Hydrogen bonding" applicable to this article? hydrophilic molecules philic organic acetic hydro acid common chemistry chem igoc ucla harding edu 2A,B,C). Such a shift in balance can be seen as a jump between free energy landscape minima, and could occur, for example, at a critical concentration [58], or pH [55], or at a temperature sufficiently high to overcome kinetic barriers between the native and amyloid minima [46]. The inclusion of the HP term in Eq. Hydrophobic Interactions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Hence the hydrophobic effect is essential to life. Northern Arizona University: Polar and Non-Polar Molecules. Nevertheless, hydrogen bonding does play an important role in protein folding since highly polar sequences can fold to form -helices, and side-chain only molecular dynamics simulations fail to capture crucial aspects of protein folding [24]. These results characterise the nature of the interactions that determine the competition between folding and misfolding of proteins by revealing that the stability of native proteins is primarily determined by hydrophobic interactions between side-chains, while the stability of amyloid fibrils depends more on backbone intermolecular hydrogen bonding interactions. Have you ever filled up a glass of water to the very top and then slowly added a few more drops? [21], which we refer to as the SJKG matrix, explicitly includes effects due to chain connectivity. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. When existing parallel and antiparallel -sheet propensity scales [31] are converted into free energies (Table 2, column 5 and 6 respectively), grouped into polar and non-polar terms and then separately shifted to have zero mean, thus removing the average hydrophobic (hydrophilic) cost (gain) to water of forming a sheet (the values are +0.32 (0.51 ) and +0.34 (0.25 ) for parallel and antiparallel -sheets respectively), the remainder correlates extremely well with (correlation coefficients of 0.96 and 0.97 for parallel and antiparallel -sheets respectively, Fig. These two-body interactions are described by three interaction matrices, , and , with the following properties: if and are both hydrophobic residues and topological neighbours, and otherwise; if either or is a hydrophobic residue, and are topological neighbours, and otherwise; if and can both form backbone hydrogen bonds and are topological neighbours, otherwise . Non-polar molecules don't have differently charged ends and so can't break the hydrogen bonds of the water molecules. The H+ ions can combine with the OH ions, limiting the increase in pH. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Physical Chemistry for the Life Sciences. The water molecules that form the "cage" (or clathrate) have restricted mobility. Firstly, the value of is only slightly affected by considering amino acids such as Proline and Alanine to be hydrophilic rather than hydrophobic. Increased energy disrupts the hydrogen bonds between water molecules. [1] but have different amino acid interaction matrices , according to Eq. Sausalito, CA: Edwards Brothers, Inc. 2005. [6] describing protein folding and misfolding is analogous to that of a spin glass model in which competition between conflicting interactions leads to a rugged free energy landscape [41]. American chemist Walter Kauzmann discovered that nonpolar substances like fat molecules tend to clump up together rather than distributing itself in a water medium, because this allow the fat molecules to have minimal contact with water. Since the Hamiltonian maps the sequence space on to the structure space, as the weights , and change so too does the shape of the resulting structure. This situation occurs as the competing processes of protein folding and misfolding are finely tuned in terms of their free energies. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.11). The twenty one-body terms, , were determined by performing a simulated annealing minimisation in MATLAB. This approach suggests that while hydrophobicity, hydrogen bonding and the formation of secondary structure are important to both processes, the balance between hydrophobicity and hydrogen bonding is remarkably different in the two regimes. Column chromatography with a hydrophobic stationary phase such as phenyl-sepharose will cause more hydrophobic proteins to travel more slowly, while less hydrophobic ones elute from the column sooner. One of the most studied matrices of this type has been reported by Miyazawa and Jernigan [5]. 2022 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved.
A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. Water molecules that are distorted by the presence of the hydrophobe will make new hydrogen bonds and form an ice-like cage structure called a clathrate cage around the hydrophobe. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. If you're seeing this message, it means we're having trouble loading external resources on our website. This assumption is supported by the observation that the PASTA matrices are highly successful at predicting the portions of a polypeptide sequence that stabilise the core regions of experimentally determined amyloid fibrils and the intra-sheet registry of the -sheets [9]. No, Is the Subject Area "Hydrogen bonding" applicable to this article? hydrophilic molecules philic organic acetic hydro acid common chemistry chem igoc ucla harding edu 2A,B,C). Such a shift in balance can be seen as a jump between free energy landscape minima, and could occur, for example, at a critical concentration [58], or pH [55], or at a temperature sufficiently high to overcome kinetic barriers between the native and amyloid minima [46]. The inclusion of the HP term in Eq. Hydrophobic Interactions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Hence the hydrophobic effect is essential to life. Northern Arizona University: Polar and Non-Polar Molecules. Nevertheless, hydrogen bonding does play an important role in protein folding since highly polar sequences can fold to form -helices, and side-chain only molecular dynamics simulations fail to capture crucial aspects of protein folding [24]. These results characterise the nature of the interactions that determine the competition between folding and misfolding of proteins by revealing that the stability of native proteins is primarily determined by hydrophobic interactions between side-chains, while the stability of amyloid fibrils depends more on backbone intermolecular hydrogen bonding interactions. Have you ever filled up a glass of water to the very top and then slowly added a few more drops? [21], which we refer to as the SJKG matrix, explicitly includes effects due to chain connectivity. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. When existing parallel and antiparallel -sheet propensity scales [31] are converted into free energies (Table 2, column 5 and 6 respectively), grouped into polar and non-polar terms and then separately shifted to have zero mean, thus removing the average hydrophobic (hydrophilic) cost (gain) to water of forming a sheet (the values are +0.32 (0.51 ) and +0.34 (0.25 ) for parallel and antiparallel -sheets respectively), the remainder correlates extremely well with (correlation coefficients of 0.96 and 0.97 for parallel and antiparallel -sheets respectively, Fig. These two-body interactions are described by three interaction matrices, , and , with the following properties: if and are both hydrophobic residues and topological neighbours, and otherwise; if either or is a hydrophobic residue, and are topological neighbours, and otherwise; if and can both form backbone hydrogen bonds and are topological neighbours, otherwise . Non-polar molecules don't have differently charged ends and so can't break the hydrogen bonds of the water molecules. The H+ ions can combine with the OH ions, limiting the increase in pH. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Physical Chemistry for the Life Sciences. The water molecules that form the "cage" (or clathrate) have restricted mobility. Firstly, the value of is only slightly affected by considering amino acids such as Proline and Alanine to be hydrophilic rather than hydrophobic. Increased energy disrupts the hydrogen bonds between water molecules. [1] but have different amino acid interaction matrices , according to Eq. Sausalito, CA: Edwards Brothers, Inc. 2005. [6] describing protein folding and misfolding is analogous to that of a spin glass model in which competition between conflicting interactions leads to a rugged free energy landscape [41]. American chemist Walter Kauzmann discovered that nonpolar substances like fat molecules tend to clump up together rather than distributing itself in a water medium, because this allow the fat molecules to have minimal contact with water. Since the Hamiltonian maps the sequence space on to the structure space, as the weights , and change so too does the shape of the resulting structure. This situation occurs as the competing processes of protein folding and misfolding are finely tuned in terms of their free energies. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.11). The twenty one-body terms, , were determined by performing a simulated annealing minimisation in MATLAB. This approach suggests that while hydrophobicity, hydrogen bonding and the formation of secondary structure are important to both processes, the balance between hydrophobicity and hydrogen bonding is remarkably different in the two regimes. Column chromatography with a hydrophobic stationary phase such as phenyl-sepharose will cause more hydrophobic proteins to travel more slowly, while less hydrophobic ones elute from the column sooner. One of the most studied matrices of this type has been reported by Miyazawa and Jernigan [5]. 2022 Leaf Group Ltd. / Leaf Group Media, All Rights Reserved.  We also note that in the -sheets of globular proteins, the effects of backbone hydrogen bonding tends to be averaged out in Eq. Waters cohesive forces allow for the property of surface tension. This is because the water molecules are attracted to the straw and therefore adhere to it.
We also note that in the -sheets of globular proteins, the effects of backbone hydrogen bonding tends to be averaged out in Eq. Waters cohesive forces allow for the property of surface tension. This is because the water molecules are attracted to the straw and therefore adhere to it.  Blood Flow and Blood Pressure Regulation, 22.2. https://doi.org/10.1371/journal.pcbi.1002169.t001. Previous work has shown that one-body components of the MJ matrix, known as q-values, are closely related to the interactions governing secondary structure formation [6]. These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) (Figure 2.8 a). cellulose hydrophobic hydrophilic adapted interconversions polymorphs hypothetical synthase representing This effect is even more apparent in the simpler case of the one-body components of the parallel and antiparallel PASTA matrices (Table 2, parallel and antiparallel respectively). This interpretation implies that globular proteins are stabilised mainly by side-chain hydrophobic interactions [6] since the sum of all H-H, H-P and P-P contacts captures the overall distribution of contact free energies extremely well (Fig. This result suggests that the segregation of hydrophobic and polar residues is not very important in -sheet formation and could lead to solvent exposed non-polar side-chains in prefibrillar aggregates, a feature that has been suggested to be closely linked to cytotoxicity [30]. However, a knowledge-based pair potential for describing amino acid interactions in the native folds of globular proteins developed by Skolnick, et al. acid: a substance that donates hydrogen ions and therefore lowers pH, adhesion: the attraction between water molecules and molecules of a different substance, base: a substance that absorbs hydrogen ions and therefore raises pH, buffer: a solution that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions, cohesion: the intermolecular forces between water molecules caused by the polar nature of water; creates surface tension, evaporation: the release of water molecules from liquid water to form water vapor, hydrophilic: describes a substance that dissolves in water; water-loving, hydrophobic: describes a substance that does not dissolve in water; water-fearing, litmus paper: filter paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, pH scale: a scale ranging from 0 to 14 that measures the approximate concentration of hydrogen ions of a substance, solvent: a substance capable of dissolving another substance, surface tension: the cohesive force at the surface of a body of liquid that prevents the molecules from separating, temperature: a measure of molecular motion, Humphrey, W., Dalke, A. and Schulten, K., VMDVisual Molecular Dynamics, J. Molec. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. the 210 independent elements in a symmetric matrix). 1B,C). We also note that the MJ matrix is calculated by using the quasi-chemical approximation in which protein residues are assumed to be in equilibrium with the solvent. Hydrogen bonds are not readily formed with nonpolar substances like oils and fats . Buffers are important in biological systems because of their ability to maintain constant pH conditions. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Major advances in establishing the interactions that drive the folding process have been made by analysing the structures in the Protein Data Bank (PDB), and particularly by examining the frequency with which contacts between the different types of amino acid residues occur [5]. hydrophilic hydrophobic hydrophilic hydrophobic (E,F) Single Gaussian fits to the distributions of parallel (E) and antiparallel (F) contact free energies (0.51, s.d. Yes So how is it that we can ingest or inhale acidic or basic substances and not die?
Blood Flow and Blood Pressure Regulation, 22.2. https://doi.org/10.1371/journal.pcbi.1002169.t001. Previous work has shown that one-body components of the MJ matrix, known as q-values, are closely related to the interactions governing secondary structure formation [6]. These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) (Figure 2.8 a). cellulose hydrophobic hydrophilic adapted interconversions polymorphs hypothetical synthase representing This effect is even more apparent in the simpler case of the one-body components of the parallel and antiparallel PASTA matrices (Table 2, parallel and antiparallel respectively). This interpretation implies that globular proteins are stabilised mainly by side-chain hydrophobic interactions [6] since the sum of all H-H, H-P and P-P contacts captures the overall distribution of contact free energies extremely well (Fig. This result suggests that the segregation of hydrophobic and polar residues is not very important in -sheet formation and could lead to solvent exposed non-polar side-chains in prefibrillar aggregates, a feature that has been suggested to be closely linked to cytotoxicity [30]. However, a knowledge-based pair potential for describing amino acid interactions in the native folds of globular proteins developed by Skolnick, et al. acid: a substance that donates hydrogen ions and therefore lowers pH, adhesion: the attraction between water molecules and molecules of a different substance, base: a substance that absorbs hydrogen ions and therefore raises pH, buffer: a solution that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions, cohesion: the intermolecular forces between water molecules caused by the polar nature of water; creates surface tension, evaporation: the release of water molecules from liquid water to form water vapor, hydrophilic: describes a substance that dissolves in water; water-loving, hydrophobic: describes a substance that does not dissolve in water; water-fearing, litmus paper: filter paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, pH scale: a scale ranging from 0 to 14 that measures the approximate concentration of hydrogen ions of a substance, solvent: a substance capable of dissolving another substance, surface tension: the cohesive force at the surface of a body of liquid that prevents the molecules from separating, temperature: a measure of molecular motion, Humphrey, W., Dalke, A. and Schulten, K., VMDVisual Molecular Dynamics, J. Molec. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. the 210 independent elements in a symmetric matrix). 1B,C). We also note that the MJ matrix is calculated by using the quasi-chemical approximation in which protein residues are assumed to be in equilibrium with the solvent. Hydrogen bonds are not readily formed with nonpolar substances like oils and fats . Buffers are important in biological systems because of their ability to maintain constant pH conditions. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Major advances in establishing the interactions that drive the folding process have been made by analysing the structures in the Protein Data Bank (PDB), and particularly by examining the frequency with which contacts between the different types of amino acid residues occur [5]. hydrophilic hydrophobic hydrophilic hydrophobic (E,F) Single Gaussian fits to the distributions of parallel (E) and antiparallel (F) contact free energies (0.51, s.d. Yes So how is it that we can ingest or inhale acidic or basic substances and not die?  This means that ice floats on the surface of a body of water (Figure 2.8 b). Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! All of these unique properties of water are important in the chemistry of living organisms. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems. More bonds are broken than are formed. Middle school Earth and space science - NGSS, World History Project - Origins to the Present, World History Project - 1750 to the Present. [17] In the case of larger nonpolar molecules, the reorientational and translational motion of the water molecules in the solvation shell may be restricted by a factor of two to four; thus, at 25C the reorientational correlation time of water increases from 2 to 4-8 picoseconds. hydrophobic hydrogen phthalate vitamin parts hydrophilic methyl indicate problems practice mol problem chemistry organic molecular Interaction parameters to describe the folding process are usually defined by considering a subspace that includes the regions of conformational space corresponding to the native states of globular proteins [19]. In addition, the type of amino acid and specific sequence patterns have varying degrees of globularity [18] or aggregation propensity [16] with certain amino acids, notably proline, appearing much more frequently in globular proteins than in the core region of amyloid fibrils [9].
This means that ice floats on the surface of a body of water (Figure 2.8 b). Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! All of these unique properties of water are important in the chemistry of living organisms. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems. More bonds are broken than are formed. Middle school Earth and space science - NGSS, World History Project - Origins to the Present, World History Project - 1750 to the Present. [17] In the case of larger nonpolar molecules, the reorientational and translational motion of the water molecules in the solvation shell may be restricted by a factor of two to four; thus, at 25C the reorientational correlation time of water increases from 2 to 4-8 picoseconds. hydrophobic hydrogen phthalate vitamin parts hydrophilic methyl indicate problems practice mol problem chemistry organic molecular Interaction parameters to describe the folding process are usually defined by considering a subspace that includes the regions of conformational space corresponding to the native states of globular proteins [19]. In addition, the type of amino acid and specific sequence patterns have varying degrees of globularity [18] or aggregation propensity [16] with certain amino acids, notably proline, appearing much more frequently in globular proteins than in the core region of amyloid fibrils [9].  This means these materials, made up of non-polar molecules, are hydrophobic or water-repellent. Temperature is a measure of the motion (kinetic energy) of molecules.
This means these materials, made up of non-polar molecules, are hydrophobic or water-repellent. Temperature is a measure of the motion (kinetic energy) of molecules.  hydrophobic hydrophilic zeolite Water has many properties that are critical to maintaining life. here. These nonpolar compounds are hydrophobic (water-fearing) and will not dissolve in water. Oxford, UK: Oxford University Press. The water molecules form comparatively weak intermolecular bonds called hydrogen bonds. Skolnick, et al. Conversely, bases are those substances that readily donate OH. hydrophilic hydrophobic pva emulsion Upon folding, the protein minimises the free energy of the protein-water system by clustering hydrophobic groups and forming intramolecular hydrogen bonds in the globular interior. Tearing down a portion of the clathrate cage will cause the entropy to increase ( \( \Delta S \) is positive), since forming it decreases the entropy. Legal. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A positive \(\Delta G\) indicates that the mixing of the hydrophobe and water molecules is not spontaneous. [4] into Eq. Recent experimental evidence supports this interpretation of protein folding and misfolding. The resulting parallel strand and antiparallel strand interaction free energy matrices (referred to here as parallel and antiparallel respectively) are shown in Fig. Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. However, the amino acid sequences of individual peptides and proteins influence their specific propensity to aggregate [16], [17], and to form self-complementary side-chain packing interfaces between adjacent -sheets in the fibrils [15], [27], [28]. In the solvation shell of small nonpolar particles, the restriction amounts to some 10%. This approach enables us to analyse quantitatively the relative importance of local and non-local interactions in determining the folding and misfolding of proteins. The normalisation constant shifts the elements of the MJ and PASTA matrices along the free energy axis thus allowing comparison of , and between different matrices. Furthermore, if we adopt the partitioning suggested by Li, et al. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. This orientation makes the system (hydrophobe) more structured with an decrease of the total entropy of the system; therefore \( \Delta S < 0\). Ions are hydrophilic or attracted to water molecules because the water molecules are polar, with a negative charge at one end and a positive charge at the other end. This ratio was corroborated by decomposing three recent pairwise contact potentials for the native states of globular proteins [21][23] which gave a similar result ( values are 0.4 [21], 0.7 [22], 0.73 [23] and on average). Hence, we suggest that the one-body free energy terms, , correspond to a stabilisation of the native or fibrillar state through a competition between hydrophilicity and the formation of hydrogen bonded secondary structure. The end of the water molecule with the oxygen atom is negatively charged while the hydrogen atom end is positively charged. This generic view [12] is consistent with the observation that even hydrophilic and homopolymeric sequences of amino acids can form amyloid fibrils [13]. Preface to the original textbook, by OpenStax College, 3.2 Comparing Prokaryotic and Eukaryotic Cells, 4.3 Citric Acid Cycle and Oxidative Phosphorylation, 4.5 Connections to Other Metabolic Pathways, 5.2: The Light-Dependent Reactions of Photosynthesis, 8.3 Extensions of the Laws of Inheritance, 10.2 Biotechnology in Medicine and Agriculture, 20.2 Gas Exchange across Respiratory Surfaces, 20.4 Transport of Gases in Human Bodily Fluids, 21.4. This intricate interplay of competing interactions gives rise to multiple local minima in the effective energy function of Eq. Concepts of Biology - 1st Canadian Edition by Charles Molnar and Jane Gair is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. (2) by the presence of other secondary structure motifs (-helices, -turns and coil). hydrophobic hydrophilic vs between hydrophile difference acids amino The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. On closer inspection, analysis of these interactions in the form of a histogram shows that the distribution of contact free energies determined from the Miyazawa-Jernigan (MJ) matrix (Fig. This procedure removes an average hydrophobic penalty for non-polar residues (+1.45 ) and an average hydrophilic gain for polar residues (0.07 ). Likewise, if too much OH is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. with a small unknown value of \(\Delta H\) and a large negative value of \(\Delta{S} \), the value of \(\Delta G\) will turn out to be positive. Since , and are all binary matrices, it is straightforward to quantify the marginal effect of each of the regressors in our general linear model from the values of their coefficients , and . By virtue of using native reference states, the SJKG matrix has both positive and negative side-chain interaction free energies and is similar in this way to the PASTA matrices (Fig. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. We find that the minima of the protein free energy landscape for folding and misfolding tend to be respectively dominated by hydrophobic and by hydrogen bonding interactions. A water molecule is formed from two hydrogen atoms and an oxygen atom linked by two polar covalent bonds. Is the Subject Area "Free energy" applicable to this article? Cells no longer function properly, and proteins will break down. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. This suggests that the monomer-monomer association energy is a linear decreasing function of denaturant concentration under mildly denaturing conditions. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Competing interests: The authors have declared that no competing interests exist. The SJKG matrix also has a mean free energy of approximately zero (0.08 ) like the PASTA matrices (0.51 and 0.13 for parallel and antiparallel respectively, Fig. Since proline cannot form inter-molecular backbone hydrogen bonds this observation suggests that the stabilisation of -sheets arises mainly from the dominance of backbone hydrogen bonding, with hydrophobic interactions (Fig. This is observed when water climbs up a straw placed in a glass of water. [13][14], In biochemistry, the hydrophobic effect can be used to separate mixtures of proteins based on their hydrophobicity. In our initial classification of hydrophobic and hydrophilic residues [11], the ratios between the hydrogen bonding and hydrophobic terms, , are 0.48, 1.59 and 1.39 for the MJ, parallel and antiparallel PASTA matrices respectively (Table 1).
hydrophobic hydrophilic zeolite Water has many properties that are critical to maintaining life. here. These nonpolar compounds are hydrophobic (water-fearing) and will not dissolve in water. Oxford, UK: Oxford University Press. The water molecules form comparatively weak intermolecular bonds called hydrogen bonds. Skolnick, et al. Conversely, bases are those substances that readily donate OH. hydrophilic hydrophobic pva emulsion Upon folding, the protein minimises the free energy of the protein-water system by clustering hydrophobic groups and forming intramolecular hydrogen bonds in the globular interior. Tearing down a portion of the clathrate cage will cause the entropy to increase ( \( \Delta S \) is positive), since forming it decreases the entropy. Legal. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. A positive \(\Delta G\) indicates that the mixing of the hydrophobe and water molecules is not spontaneous. [4] into Eq. Recent experimental evidence supports this interpretation of protein folding and misfolding. The resulting parallel strand and antiparallel strand interaction free energy matrices (referred to here as parallel and antiparallel respectively) are shown in Fig. Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. However, the amino acid sequences of individual peptides and proteins influence their specific propensity to aggregate [16], [17], and to form self-complementary side-chain packing interfaces between adjacent -sheets in the fibrils [15], [27], [28]. In the solvation shell of small nonpolar particles, the restriction amounts to some 10%. This approach enables us to analyse quantitatively the relative importance of local and non-local interactions in determining the folding and misfolding of proteins. The normalisation constant shifts the elements of the MJ and PASTA matrices along the free energy axis thus allowing comparison of , and between different matrices. Furthermore, if we adopt the partitioning suggested by Li, et al. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. This orientation makes the system (hydrophobe) more structured with an decrease of the total entropy of the system; therefore \( \Delta S < 0\). Ions are hydrophilic or attracted to water molecules because the water molecules are polar, with a negative charge at one end and a positive charge at the other end. This ratio was corroborated by decomposing three recent pairwise contact potentials for the native states of globular proteins [21][23] which gave a similar result ( values are 0.4 [21], 0.7 [22], 0.73 [23] and on average). Hence, we suggest that the one-body free energy terms, , correspond to a stabilisation of the native or fibrillar state through a competition between hydrophilicity and the formation of hydrogen bonded secondary structure. The end of the water molecule with the oxygen atom is negatively charged while the hydrogen atom end is positively charged. This generic view [12] is consistent with the observation that even hydrophilic and homopolymeric sequences of amino acids can form amyloid fibrils [13]. Preface to the original textbook, by OpenStax College, 3.2 Comparing Prokaryotic and Eukaryotic Cells, 4.3 Citric Acid Cycle and Oxidative Phosphorylation, 4.5 Connections to Other Metabolic Pathways, 5.2: The Light-Dependent Reactions of Photosynthesis, 8.3 Extensions of the Laws of Inheritance, 10.2 Biotechnology in Medicine and Agriculture, 20.2 Gas Exchange across Respiratory Surfaces, 20.4 Transport of Gases in Human Bodily Fluids, 21.4. This intricate interplay of competing interactions gives rise to multiple local minima in the effective energy function of Eq. Concepts of Biology - 1st Canadian Edition by Charles Molnar and Jane Gair is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. (2) by the presence of other secondary structure motifs (-helices, -turns and coil). hydrophobic hydrophilic vs between hydrophile difference acids amino The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. On closer inspection, analysis of these interactions in the form of a histogram shows that the distribution of contact free energies determined from the Miyazawa-Jernigan (MJ) matrix (Fig. This procedure removes an average hydrophobic penalty for non-polar residues (+1.45 ) and an average hydrophilic gain for polar residues (0.07 ). Likewise, if too much OH is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. with a small unknown value of \(\Delta H\) and a large negative value of \(\Delta{S} \), the value of \(\Delta G\) will turn out to be positive. Since , and are all binary matrices, it is straightforward to quantify the marginal effect of each of the regressors in our general linear model from the values of their coefficients , and . By virtue of using native reference states, the SJKG matrix has both positive and negative side-chain interaction free energies and is similar in this way to the PASTA matrices (Fig. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. We find that the minima of the protein free energy landscape for folding and misfolding tend to be respectively dominated by hydrophobic and by hydrogen bonding interactions. A water molecule is formed from two hydrogen atoms and an oxygen atom linked by two polar covalent bonds. Is the Subject Area "Free energy" applicable to this article? Cells no longer function properly, and proteins will break down. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. This suggests that the monomer-monomer association energy is a linear decreasing function of denaturant concentration under mildly denaturing conditions. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Competing interests: The authors have declared that no competing interests exist. The SJKG matrix also has a mean free energy of approximately zero (0.08 ) like the PASTA matrices (0.51 and 0.13 for parallel and antiparallel respectively, Fig. Since proline cannot form inter-molecular backbone hydrogen bonds this observation suggests that the stabilisation of -sheets arises mainly from the dominance of backbone hydrogen bonding, with hydrophobic interactions (Fig. This is observed when water climbs up a straw placed in a glass of water. [13][14], In biochemistry, the hydrophobic effect can be used to separate mixtures of proteins based on their hydrophobicity. In our initial classification of hydrophobic and hydrophilic residues [11], the ratios between the hydrogen bonding and hydrophobic terms, , are 0.48, 1.59 and 1.39 for the MJ, parallel and antiparallel PASTA matrices respectively (Table 1).