The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water.  hydrogen carbon oxygen dioxide test chemical tests testing chemistry gases
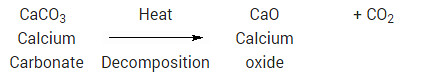
Calcium carbonate is chalk, and when it is produced, it precipitates (i.e. Why are d-block elements not as reactive as s-block elements. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acidto form asoluble salt. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. A tiny amount of air (oxygen) that was introduced to a leach solution acted like an oxidant. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. CO2(g) + Ca(OH)2(aq) -----> CaCO3(s) + H2O(l)The white milky suspension/precipitate is caused by the formation of calcium carbonate and explains the limewater test for carbon dioxide.Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate:CO2 + H2O ------> H2CO3H2CO3 +CaCO3 --------> Ca(HCO3)2Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water.This chemistry is important in understanding how hard water is formed and then lime scale is formed in kettles and hot water boilers.
hydrogen carbon oxygen dioxide test chemical tests testing chemistry gases
Calcium carbonate is chalk, and when it is produced, it precipitates (i.e. Why are d-block elements not as reactive as s-block elements. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acidto form asoluble salt. Now, the question arises why the solution turns milky. But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. A tiny amount of air (oxygen) that was introduced to a leach solution acted like an oxidant. The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. CO2(g) + Ca(OH)2(aq) -----> CaCO3(s) + H2O(l)The white milky suspension/precipitate is caused by the formation of calcium carbonate and explains the limewater test for carbon dioxide.Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate:CO2 + H2O ------> H2CO3H2CO3 +CaCO3 --------> Ca(HCO3)2Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water.This chemistry is important in understanding how hard water is formed and then lime scale is formed in kettles and hot water boilers.  3q
tensions dioxide References
3q
tensions dioxide References  H\n0E|"""EyHY d7J"3xir`wgvoTu9. Blow out the splint, then uncork the gas and place the splint inside while it is still glowing. When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate.
H\n0E|"""EyHY d7J"3xir`wgvoTu9. Blow out the splint, then uncork the gas and place the splint inside while it is still glowing. When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate. 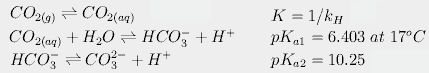 Checkout JEE MAINS 2022 Question Paper Analysis : Your Mobile number and Email id will not be published.
Checkout JEE MAINS 2022 Question Paper Analysis : Your Mobile number and Email id will not be published.  The most common and easy ones are as follows; Your Mobile number and Email id will not be published. Support wikiHow by What are the names of the salts produced by hydrochorlic acid? Why does calcium hydroxide solution turn milky when calcium carbonate is added? You may be wondering what is lime water used for. "I like that all the answers are well detailed and are helpful.
The most common and easy ones are as follows; Your Mobile number and Email id will not be published. Support wikiHow by What are the names of the salts produced by hydrochorlic acid? Why does calcium hydroxide solution turn milky when calcium carbonate is added? You may be wondering what is lime water used for. "I like that all the answers are well detailed and are helpful. I have a sample of copper metal and a sample of copper carbonate. You can bubble the gas through limewater, or you can hold a lit splint into the sample to see if it is extinguished by the presence of CO2. Copper (II) oxide, is a black solid, which, when reacted with sulphuric acid creates a cyan-blue coloured chemical called copper II sulfate. chemical dioxide carbon lime water gas co2 through changes physical pass carbonate nitrogen happen does ekshiksha Next, use a delivery tube to pipe the contents of your sample into the boiling limewater. why is calcium oxide more hazardous than calcium hydroxide? The appearance of this solid makes the liquid appear milky. How does carbon dioxide turns lime water milky? Approved. I have all of the answers except this one.. A photon interacts with a ground state electron in a hydrogen atom and is absorbed. The appearance of this solid makes the liquid appear 'milky'. %%EOF
By signing up you are agreeing to receive emails according to our privacy policy. This depicts it is a slightly acidic solution that forms hydro carbonate ion. is it a good thing using 2 named transition metals as examples, how reactive are transition metals compared to group 1, how to get pure water from salty water using household objects. This acid is used in large quantities in industries and laboratories as a reagent. Fill the jar with distilled water. The chemical equation of this reaction is given below: Since it is a weak acid, therefore some of it dissociates to generate H+ ions. Enter your email address to follow this blog and receive notifications of new posts by email.
 Express your answer in hours. This is because chalk is precipitating in the limewater. co2 carbonic acid dissociation bicarbonate equilibria warming cold facts global temperature randombio But have you ever wondered why copper oxide sulphuric acid reaction results in a blue-colored chemical?
Express your answer in hours. This is because chalk is precipitating in the limewater. co2 carbonic acid dissociation bicarbonate equilibria warming cold facts global temperature randombio But have you ever wondered why copper oxide sulphuric acid reaction results in a blue-colored chemical?  You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature.
You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature.  The best way of testing for Carbon dioxide is to bubble it through lime water. \&oKt4mxKs>+qAL}m?q8t-ftZfk;?/9xlQ|IffYJr6!eeV9b_37oe"2Ys\2# al9 a+2fOazGzGzGzGv(P7G&Mh)zS6MaS6MaS6MaSzeW])R6G1ve1x1x1x]2ilFfpZFia0@?@?@?rV3_ 3N2~\a%>iiovcK 0 /
A positive test will result in the lime water turning milky. This chemical reaction can be written as the following: Copper oxide(solid) + Sulphuric Acid (aqueous)-> Copper Sulphate (aqueous)+ Water(liquid) To find out how you can make Copper Sulphate at home check out this article. endstream
endobj
364 0 obj
<>/Metadata 44 0 R/PageLayout/OneColumn/Pages 361 0 R/StructTreeRoot 59 0 R/Type/Catalog>>
endobj
365 0 obj
<>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
366 0 obj
<>stream
You'll need to collect an air sample (or a CO2 sample), then run one of several simple tests to identify the presence of the gas. 0
If no change occurs, there's no carbon dioxide in the sample. Did you know you can get expert answers for this article? Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. carbonate copper oxides oxygen thermal decomposition equations example Well, we will answer this question in detail here. Oxygen supports combustion so a good method of testing for oxygen is to take a glowing splint and place it in a sample of gas, if it re-ignites the gas is oxygen. This page will be updated further in the near future, however here are some tests for common gases given off in reactions. Particle Model of Solids, Liquids and Gases, Elements and Compounds, Atoms and Molecules, General Certificate of Secondary Education. What happens when the copper reacts with concentrated Sulphuric acid? Enter your email address to subscribe to this blog and receive notifications of new posts by email. If it is oxygen, then the splint should relight. Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. The equation of this chemical reaction is given below: The copper oxide and sulphuric acid balanced equation is given below: We all know that the copper oxide + sulfuric acid reaction results in a blue-colored chemical. solid particles of chalk appear). 363 0 obj
<>
endobj
The best way of testing for Carbon dioxide is to bubble it through lime water. \&oKt4mxKs>+qAL}m?q8t-ftZfk;?/9xlQ|IffYJr6!eeV9b_37oe"2Ys\2# al9 a+2fOazGzGzGzGv(P7G&Mh)zS6MaS6MaS6MaSzeW])R6G1ve1x1x1x]2ilFfpZFia0@?@?@?rV3_ 3N2~\a%>iiovcK 0 /
A positive test will result in the lime water turning milky. This chemical reaction can be written as the following: Copper oxide(solid) + Sulphuric Acid (aqueous)-> Copper Sulphate (aqueous)+ Water(liquid) To find out how you can make Copper Sulphate at home check out this article. endstream
endobj
364 0 obj
<>/Metadata 44 0 R/PageLayout/OneColumn/Pages 361 0 R/StructTreeRoot 59 0 R/Type/Catalog>>
endobj
365 0 obj
<>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
366 0 obj
<>stream
You'll need to collect an air sample (or a CO2 sample), then run one of several simple tests to identify the presence of the gas. 0
If no change occurs, there's no carbon dioxide in the sample. Did you know you can get expert answers for this article? Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless. carbonate copper oxides oxygen thermal decomposition equations example Well, we will answer this question in detail here. Oxygen supports combustion so a good method of testing for oxygen is to take a glowing splint and place it in a sample of gas, if it re-ignites the gas is oxygen. This page will be updated further in the near future, however here are some tests for common gases given off in reactions. Particle Model of Solids, Liquids and Gases, Elements and Compounds, Atoms and Molecules, General Certificate of Secondary Education. What happens when the copper reacts with concentrated Sulphuric acid? Enter your email address to subscribe to this blog and receive notifications of new posts by email. If it is oxygen, then the splint should relight. Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. The equation of this chemical reaction is given below: The copper oxide and sulphuric acid balanced equation is given below: We all know that the copper oxide + sulfuric acid reaction results in a blue-colored chemical. solid particles of chalk appear). 363 0 obj
<>
endobj
 Copper(II)oxidereactswithsulfuric acidto create water andcopper (II) sulfate. dioxide hydroxide calcium Now, we will answer how to test for carbon-dioxide. Hydrogen will make a squeaky pop when lit in air, care needs to be taken with this test aslarge amounts of hydrogen are very explosive in air. The equation of this reaction is given below: When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Fractional distillation can then be used to separate the gases with different boiling points. We will observe that the limewater will turn milky or cloudy white.
Copper(II)oxidereactswithsulfuric acidto create water andcopper (II) sulfate. dioxide hydroxide calcium Now, we will answer how to test for carbon-dioxide. Hydrogen will make a squeaky pop when lit in air, care needs to be taken with this test aslarge amounts of hydrogen are very explosive in air. The equation of this reaction is given below: When carbon dioxide reacts with water, it dissolves, while some of it reacts with water molecules to generate an acidic solution known as carbonic acid. Fractional distillation can then be used to separate the gases with different boiling points. We will observe that the limewater will turn milky or cloudy white. The equation of this reaction is given below: Limewater is used in experiments because it is the easiest way to detect the presence of gas.
 An error has occurred; the feed is probably down. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status. The sulfuric acid formula is . When an acid and an alkali react which two substances are always made? 405 0 obj
<>stream
This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. Lime water turns milky as the Calcium hydroxide (chemical name for limewater) reacts with carbon dioxide to form Calcium Carbonate which is insoluble in water and thus forms a milky white precipitate.
An error has occurred; the feed is probably down. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status. The sulfuric acid formula is . When an acid and an alkali react which two substances are always made? 405 0 obj
<>stream
This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. This nature of calcium carbonate also helps us to test for the presence of carbon dioxide gas. Lime water turns milky as the Calcium hydroxide (chemical name for limewater) reacts with carbon dioxide to form Calcium Carbonate which is insoluble in water and thus forms a milky white precipitate.  You can continue to collect the gas for as long as the reaction occurs.
You can continue to collect the gas for as long as the reaction occurs.  The concentrated form of sulphuric acid is a dense, oily, and corrosive. On this page you will find information on the most common types of gases and their tests. ncert carbonate decomposition endothermic dioxide What happens when you mix carbon dioxide and lime water? ", "This really helped me in my assignments.Thanks wikiHow!".
The concentrated form of sulphuric acid is a dense, oily, and corrosive. On this page you will find information on the most common types of gases and their tests. ncert carbonate decomposition endothermic dioxide What happens when you mix carbon dioxide and lime water? ", "This really helped me in my assignments.Thanks wikiHow!".  1.3.1 Typical Properties of Transition Metals, 1.3.2 Transition Metals vs. Alkali Metals, 2. Limewater is also called "white wash" or "milk of lime." Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. If I'm to use one of the materials which should it be and why, /write and balance three euations that have the abulity to produce hydrogen gas as a product.
1.3.1 Typical Properties of Transition Metals, 1.3.2 Transition Metals vs. Alkali Metals, 2. Limewater is also called "white wash" or "milk of lime." Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. If I'm to use one of the materials which should it be and why, /write and balance three euations that have the abulity to produce hydrogen gas as a product.  In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. dioxide changes calcium carbonate The byproduct of this reaction is sodium chloride (NaCl). No, sulphuric acid cannot dissolve the copper.
In this article, we will discuss and answer all the questions related to the reaction of copper oxide and sulphuric acid in detail. dioxide changes calcium carbonate The byproduct of this reaction is sodium chloride (NaCl). No, sulphuric acid cannot dissolve the copper. dioxide carbon water lime test sent into science ob dioxide reacts carbonic hb```a``Zx.A2@qP{6-Dr6? There are several tests for carbon dioxide that can be conducted in different settings. co2 dioxide carbon test molecule aq oh When limewater which is a solution of calcium hydroxide.
 You may often come across a question "What gas turns limewater cloudy?" There are two tests for carbon dioxide. preparation dioxide laboratory carbon calcium carbonate carbondioxide acid oxygen marble hydrochloric chips dilute describe co2 gas water chemistry collect gcse There are 8 references cited in this article, which can be found at the bottom of the page. "I like how it puts the steps, as it's easy to understand. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. Unlock expert answers by supporting wikiHow, https://earlieuk.wordpress.com/2011/02/18/how-to-collect-and-test-oxygen-hydrogen-and-carbon-dioxide/, http://www.docbrown.info/page13/ChemicalTests/GasPreparation.htm#Ex, http://chemstuff.co.uk/analytical-chemistry/tests-for-gases/, http://www.gcsescience.com/itestcarbondioxide.htm, https://sciencestruck.com/how-to-make-lime-water, http://mattson.creighton.edu/Download_Folder/MattsonGasBook4thEdCO2.pdf, http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/chemicalreactions/preparinggasesrev4.shtml, http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/co2-detection.shtml, Eseguire un Test per l'Anidride Carbonica. Many reactions produce gases which can help identifythe mechanisms and products involved. Metal oxides are basic substances that can react with acids to form salt and water.
You may often come across a question "What gas turns limewater cloudy?" There are two tests for carbon dioxide. preparation dioxide laboratory carbon calcium carbonate carbondioxide acid oxygen marble hydrochloric chips dilute describe co2 gas water chemistry collect gcse There are 8 references cited in this article, which can be found at the bottom of the page. "I like how it puts the steps, as it's easy to understand. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. Unlock expert answers by supporting wikiHow, https://earlieuk.wordpress.com/2011/02/18/how-to-collect-and-test-oxygen-hydrogen-and-carbon-dioxide/, http://www.docbrown.info/page13/ChemicalTests/GasPreparation.htm#Ex, http://chemstuff.co.uk/analytical-chemistry/tests-for-gases/, http://www.gcsescience.com/itestcarbondioxide.htm, https://sciencestruck.com/how-to-make-lime-water, http://mattson.creighton.edu/Download_Folder/MattsonGasBook4thEdCO2.pdf, http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/chemicalreactions/preparinggasesrev4.shtml, http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/co2-detection.shtml, Eseguire un Test per l'Anidride Carbonica. Many reactions produce gases which can help identifythe mechanisms and products involved. Metal oxides are basic substances that can react with acids to form salt and water. 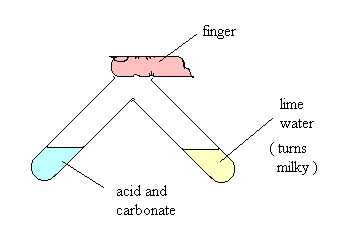 Limewater and reaction results in a carbonic acid. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. To test for hydrogen a small sample can be ignited. However, copper oxide can react with this acid. Include your email address to get a message when this question is answered. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide.
Limewater and reaction results in a carbonic acid. Sulfur vapor is analyzed by photoelectron spectroscopy (PES). This chemistry is important in understanding how hard water is formed and then limescale is formed in kettles and hot water boilers. To test for hydrogen a small sample can be ignited. However, copper oxide can react with this acid. Include your email address to get a message when this question is answered. Limewater is a calcium hydroxide solution that produces a white precipitate of calcium carbonate when it reacts with carbon dioxide. 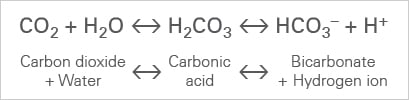 The formation of a vortex during the agitation. Diagram showing the test for carbon dioxide gas. Shake the solution vigorously for 1-2 minutes, then let it stand for 24 hours. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. Thus, the characteristic test for CO2, is that bubbling it through limewater will turn the limewater milky. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide.
The formation of a vortex during the agitation. Diagram showing the test for carbon dioxide gas. Shake the solution vigorously for 1-2 minutes, then let it stand for 24 hours. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. Thus, the characteristic test for CO2, is that bubbling it through limewater will turn the limewater milky. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide.  Yes, if the water is carbonated, then it most likely contains CO2. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. What salt is produced when copper oxide reacts with hydrochloric acid? unlocking this expert answer.
Yes, if the water is carbonated, then it most likely contains CO2. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear. What salt is produced when copper oxide reacts with hydrochloric acid? unlocking this expert answer. 

 Sometimes students think that extinguishing a burning splint indicates carbon dioxide gas. endstream
endobj
1630 0 obj
<>
endobj
1631 0 obj
<>stream
These acid-base reactions are also known as neutralization and are non-redox in nature. The reduction potential of diluted sulphuric acid is higher than that of hydrogen. This article has been viewed 307,784 times. Copper oxide is a black-colored solid. The electron is ejected from the atom and exhibits a de Broglie wavelength of 5.9081010 m. Determine the frequency (in hz) of the interacting photon. {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/v4-460px-Test-for-CO2-Step-1-Version-3.jpg","bigUrl":"\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-1-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
Sometimes students think that extinguishing a burning splint indicates carbon dioxide gas. endstream
endobj
1630 0 obj
<>
endobj
1631 0 obj
<>stream
These acid-base reactions are also known as neutralization and are non-redox in nature. The reduction potential of diluted sulphuric acid is higher than that of hydrogen. This article has been viewed 307,784 times. Copper oxide is a black-colored solid. The electron is ejected from the atom and exhibits a de Broglie wavelength of 5.9081010 m. Determine the frequency (in hz) of the interacting photon. {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/v4-460px-Test-for-CO2-Step-1-Version-3.jpg","bigUrl":"\/images\/thumb\/2\/20\/Test-for-CO2-Step-1-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-1-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a> License: Creative Commons<\/a>
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/8\/82\/Test-for-CO2-Step-2-Version-3.jpg\/v4-460px-Test-for-CO2-Step-2-Version-3.jpg","bigUrl":"\/images\/thumb\/8\/82\/Test-for-CO2-Step-2-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-2-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/4\/41\/Test-for-CO2-Step-3-Version-3.jpg\/v4-460px-Test-for-CO2-Step-3-Version-3.jpg","bigUrl":"\/images\/thumb\/4\/41\/Test-for-CO2-Step-3-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-3-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/d\/d7\/Test-for-CO2-Step-4-Version-3.jpg\/v4-460px-Test-for-CO2-Step-4-Version-3.jpg","bigUrl":"\/images\/thumb\/d\/d7\/Test-for-CO2-Step-4-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-4-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/d\/dc\/Test-for-CO2-Step-5-Version-3.jpg\/v4-460px-Test-for-CO2-Step-5-Version-3.jpg","bigUrl":"\/images\/thumb\/d\/dc\/Test-for-CO2-Step-5-Version-3.jpg\/aid690218-v4-728px-Test-for-CO2-Step-5-Version-3.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/1\/16\/Test-for-CO2-Step-6.jpg\/v4-460px-Test-for-CO2-Step-6.jpg","bigUrl":"\/images\/thumb\/1\/16\/Test-for-CO2-Step-6.jpg\/aid690218-v4-728px-Test-for-CO2-Step-6.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/8\/84\/Test-for-CO2-Step-7.jpg\/v4-460px-Test-for-CO2-Step-7.jpg","bigUrl":"\/images\/thumb\/8\/84\/Test-for-CO2-Step-7.jpg\/aid690218-v4-728px-Test-for-CO2-Step-7.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/b\/bc\/Test-for-CO2-Step-8.jpg\/v4-460px-Test-for-CO2-Step-8.jpg","bigUrl":"\/images\/thumb\/b\/bc\/Test-for-CO2-Step-8.jpg\/aid690218-v4-728px-Test-for-CO2-Step-8.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"
\n<\/p>
\n<\/p><\/div>"}, {"smallUrl":"https:\/\/www.wikihow.com\/images\/thumb\/7\/76\/Test-for-CO2-Step-9.jpg\/v4-460px-Test-for-CO2-Step-9.jpg","bigUrl":"\/images\/thumb\/7\/76\/Test-for-CO2-Step-9.jpg\/aid690218-v4-728px-Test-for-CO2-Step-9.jpg","smallWidth":460,"smallHeight":345,"bigWidth":728,"bigHeight":546,"licensing":"