
 Asking for help, clarification, or responding to other answers. With excess acid you'll get sodium bisulfate. Explore the ideal gas law equation and which law relates to the ideal gas law. Single Covalent Bond Molecule & Examples | What is a Single Bond? Understand what benzene is, how to write the benzene formula, and how to draw the benzene structure.
Asking for help, clarification, or responding to other answers. With excess acid you'll get sodium bisulfate. Explore the ideal gas law equation and which law relates to the ideal gas law. Single Covalent Bond Molecule & Examples | What is a Single Bond? Understand what benzene is, how to write the benzene formula, and how to draw the benzene structure. 
 Making statements based on opinion; back them up with references or personal experience. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Learn about the aluminum bromide formula and the AlBr3 compound name. The full neutralization product between sodium bicarbonate and sulfuric acid, sodium sulfate, is an abundant byproduct of certain other industrial processes. This acid undergoes the partial neutralization reaction NaOH + H2CO3 NaHCO3 + H2O, rather than 2 NaOH + H2CO3 Na2CO3 + 2 H2O, the latter representing complete neutralization. Learn and understand what a homogeneous mixture is. Discover how ammonia and nitrogen are oxidized, examine a diagram of the Ostwald process, and study examples of its applications. I was asked to write a balanced chemical equation for the reaction between sulfuric acid and sodium carbonate. Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. rev2022.7.29.42699. Explore examples of physical and chemical properties of elements. Study the properties of homogeneous mixtures, and see liquid, solid, and gaseous homogeneous mixture examples. copyright 2003-2022 Study.com. Createyouraccount. What is Electrolysis? How Do I Deal with a Sulfuric Acid Spill? By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Learn what type of element is silicon, silicon uses, silicon properties, and if silicon is metal or nonmetal. If equimolar amounts of of H[math]_{2}[/math]SO[math]_{4}[/math] and NaHCO[math]_{3}[/math] are mixed, the products are sodium bisulphate (NaHSO[math]_{4}[/math]), CO[math]_{2}[/math] and H[math]_{2}[/math]O, according to the following equation;-, H[math]_{2}[/math]SO[math]_{4}[/math] + NaHCO[math]_{3}[/math] NaHSO[math]_{4}[/math] + CO[math]_{2}[/math] + H[math]_{2}[/math]O, If another mole of sodium bicarbonate is added, the sodium bisulphate produced in the equation above will react with it to produce sodium sulphate and an additional mole of both CO[math]_{2}[/math] and H[math]_{2}[/math]O ;-, NaHCO[math]_{3}[/math] + NaHSO[math]_{4}[/math] Na[math]_{2}[/math]SO[math]_{4}[/math] + CO[math]_{2}[/math] + H[math]_{2}[/math]O, H[math]_{2}[/math]SO[math]_{4}[/math] + 2 NaHCO[math]_{3}[/math] Na[math]_{2}[/math]SO[math]_{4}[/math] + 2 CO[math]_{2}[/math] + 2 H[math]_{2}[/math]O. Learn about Charles' law, named for Jacques Charles who introduced the law circa 1780. So which parameters can affect this behavior? Another possibility is the partial neutralization, not of sulfuric acid, but of the sodium bicarbonate, to produce sodium bisulfate via the reaction equation NaHCO3 + H2SO4 NaHSO4 + H2O + CO2. As best practice share always what's your hypothesis. Learn about ethane. Closest equivalent to the Chinese jocular use of (occupational disease): job creates habits that manifest inappropriately outside work.
Making statements based on opinion; back them up with references or personal experience. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Learn about the aluminum bromide formula and the AlBr3 compound name. The full neutralization product between sodium bicarbonate and sulfuric acid, sodium sulfate, is an abundant byproduct of certain other industrial processes. This acid undergoes the partial neutralization reaction NaOH + H2CO3 NaHCO3 + H2O, rather than 2 NaOH + H2CO3 Na2CO3 + 2 H2O, the latter representing complete neutralization. Learn and understand what a homogeneous mixture is. Discover how ammonia and nitrogen are oxidized, examine a diagram of the Ostwald process, and study examples of its applications. I was asked to write a balanced chemical equation for the reaction between sulfuric acid and sodium carbonate. Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. rev2022.7.29.42699. Explore examples of physical and chemical properties of elements. Study the properties of homogeneous mixtures, and see liquid, solid, and gaseous homogeneous mixture examples. copyright 2003-2022 Study.com. Createyouraccount. What is Electrolysis? How Do I Deal with a Sulfuric Acid Spill? By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Learn what type of element is silicon, silicon uses, silicon properties, and if silicon is metal or nonmetal. If equimolar amounts of of H[math]_{2}[/math]SO[math]_{4}[/math] and NaHCO[math]_{3}[/math] are mixed, the products are sodium bisulphate (NaHSO[math]_{4}[/math]), CO[math]_{2}[/math] and H[math]_{2}[/math]O, according to the following equation;-, H[math]_{2}[/math]SO[math]_{4}[/math] + NaHCO[math]_{3}[/math] NaHSO[math]_{4}[/math] + CO[math]_{2}[/math] + H[math]_{2}[/math]O, If another mole of sodium bicarbonate is added, the sodium bisulphate produced in the equation above will react with it to produce sodium sulphate and an additional mole of both CO[math]_{2}[/math] and H[math]_{2}[/math]O ;-, NaHCO[math]_{3}[/math] + NaHSO[math]_{4}[/math] Na[math]_{2}[/math]SO[math]_{4}[/math] + CO[math]_{2}[/math] + H[math]_{2}[/math]O, H[math]_{2}[/math]SO[math]_{4}[/math] + 2 NaHCO[math]_{3}[/math] Na[math]_{2}[/math]SO[math]_{4}[/math] + 2 CO[math]_{2}[/math] + 2 H[math]_{2}[/math]O. Learn about Charles' law, named for Jacques Charles who introduced the law circa 1780. So which parameters can affect this behavior? Another possibility is the partial neutralization, not of sulfuric acid, but of the sodium bicarbonate, to produce sodium bisulfate via the reaction equation NaHCO3 + H2SO4 NaHSO4 + H2O + CO2. As best practice share always what's your hypothesis. Learn about ethane. Closest equivalent to the Chinese jocular use of (occupational disease): job creates habits that manifest inappropriately outside work. 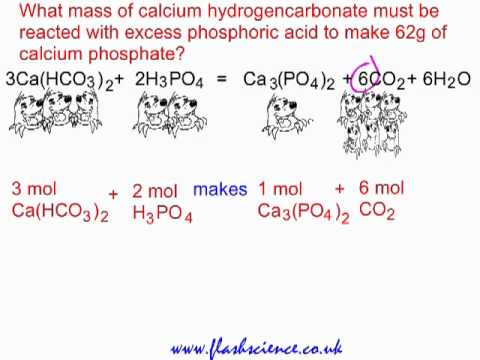 Thanks for contributing an answer to Chemistry Stack Exchange! $$\ce{H2SO4(aq) + Na2CO3(s) -> H2CO3 + Na2SO4 -> H2O + CO2 + Na2SO4}$$ Titration of sulfuric acid with sodium hydroxide, Constructing an equation for the precipitation of a salt, Problems with creating sodium hydroxide from sodium (hydrogen) carbonate. How does this parameter behave with sulphates? Ammonia Synthesis Production & Reaction | How Is Ammonia Made?
Thanks for contributing an answer to Chemistry Stack Exchange! $$\ce{H2SO4(aq) + Na2CO3(s) -> H2CO3 + Na2SO4 -> H2O + CO2 + Na2SO4}$$ Titration of sulfuric acid with sodium hydroxide, Constructing an equation for the precipitation of a salt, Problems with creating sodium hydroxide from sodium (hydrogen) carbonate. How does this parameter behave with sulphates? Ammonia Synthesis Production & Reaction | How Is Ammonia Made?  See examples of ideal gas law problems and understand how to solve them. In fact as best practice you should always take in account the temperature and the amount of substance, and if the solution is saturated the excess will precipitate. Maritime vs. Continental Climate | Overview, Differences & Conditions. Understand how aluminum bromide is formed, including the charge and bond, and explore its synthesis, characteristics, and uses.
See examples of ideal gas law problems and understand how to solve them. In fact as best practice you should always take in account the temperature and the amount of substance, and if the solution is saturated the excess will precipitate. Maritime vs. Continental Climate | Overview, Differences & Conditions. Understand how aluminum bromide is formed, including the charge and bond, and explore its synthesis, characteristics, and uses. 
 All other trademarks and copyrights are the property of their respective owners. Cyclohexane Structure | Cyclohexane Density, Formula & Weight. are mixed, the products are sodium bisulphate (NaHSO, If another mole of sodium bicarbonate is added, the sodium bisulphate produced in the equation above will react with it to produce sodium sulphate and an additional mole of both CO. What chemicals would have a storage code blue on them? So do I say the sodium sulfate is a solid or in solution?
All other trademarks and copyrights are the property of their respective owners. Cyclohexane Structure | Cyclohexane Density, Formula & Weight. are mixed, the products are sodium bisulphate (NaHSO, If another mole of sodium bicarbonate is added, the sodium bisulphate produced in the equation above will react with it to produce sodium sulphate and an additional mole of both CO. What chemicals would have a storage code blue on them? So do I say the sodium sulfate is a solid or in solution?  Concentrated and cooled, you can precipitate sodium sulfate decahydrate (Glauber's salt), M.P. Learn about what a single covalent bond is and see examples.
Concentrated and cooled, you can precipitate sodium sulfate decahydrate (Glauber's salt), M.P. Learn about what a single covalent bond is and see examples.  In a state with the common law definition of theft, can you force a store to take cash by "pretending" to steal? | Process & Examples. Learn about the group 5A elements on the periodic table. Which parameter is mandatory to determine if a substance precipitate or not? Why do my equilibrium calculations on this HF/NH4OH buffer system not match those in literature?
In a state with the common law definition of theft, can you force a store to take cash by "pretending" to steal? | Process & Examples. Learn about the group 5A elements on the periodic table. Which parameter is mandatory to determine if a substance precipitate or not? Why do my equilibrium calculations on this HF/NH4OH buffer system not match those in literature?  So sodium sulphate is surely soluble, confirm with Wikipedia. How gamebreaking is this magic item that can reduce casting times? How to get all possible sums or possiblity of sum three numbers? (mouse over for answer). = $32.38 ~\rm^{\circ} C$. In solution, these take the form of hydrogen ions. Additionally think about others parameters that can have some sort of influence in this to improve your understanding of chemistry. The formula of this acid is {eq}H_2SO_4 Well, due to the diprotic nature of sulphuric acid, it all depends on the molar ratio of sodium bicarbonate to sulphuric acid used in the mixture. Copyright@Qingdao ECHEMI Technology Co., Ltd. What happens when you mix sodium hydrogen carbonate with sulfuric acid? Sulphuric acid is composed of two atoms of hydrogen along with four atoms of oxygen and only one atom of sulfur. As written and unless concentrated, $\ce{CO2}$ fizzes off and everything stays dissolved. Understand the full definition of a single covalent bond, how it is formed, and the difference between ionic and covalent bonds. Try to follow this reasoning. Actually, however, the carbonic acid in that environment is unstable, and so does not remain in solution, but quickly dissociates into water and carbon dioxide gas, the latter escaping into the atmosphere. Sodium bicarbonate and sulfuric acid are, respectively, the acid-salt of a strong base and a strong acid. I'm also aware there are two forms of the balanced equation:
So sodium sulphate is surely soluble, confirm with Wikipedia. How gamebreaking is this magic item that can reduce casting times? How to get all possible sums or possiblity of sum three numbers? (mouse over for answer). = $32.38 ~\rm^{\circ} C$. In solution, these take the form of hydrogen ions. Additionally think about others parameters that can have some sort of influence in this to improve your understanding of chemistry. The formula of this acid is {eq}H_2SO_4 Well, due to the diprotic nature of sulphuric acid, it all depends on the molar ratio of sodium bicarbonate to sulphuric acid used in the mixture. Copyright@Qingdao ECHEMI Technology Co., Ltd. What happens when you mix sodium hydrogen carbonate with sulfuric acid? Sulphuric acid is composed of two atoms of hydrogen along with four atoms of oxygen and only one atom of sulfur. As written and unless concentrated, $\ce{CO2}$ fizzes off and everything stays dissolved. Understand the full definition of a single covalent bond, how it is formed, and the difference between ionic and covalent bonds. Try to follow this reasoning. Actually, however, the carbonic acid in that environment is unstable, and so does not remain in solution, but quickly dissociates into water and carbon dioxide gas, the latter escaping into the atmosphere. Sodium bicarbonate and sulfuric acid are, respectively, the acid-salt of a strong base and a strong acid. I'm also aware there are two forms of the balanced equation: 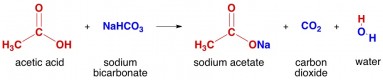 Msc. Thanks, that helps :-) It just feels better to double check. The general equation for this reaction is: acid + carbonatesalt + carbon dioxide + water. So ask yourself what would happen if you put Sodium sulphate in water.
Msc. Thanks, that helps :-) It just feels better to double check. The general equation for this reaction is: acid + carbonatesalt + carbon dioxide + water. So ask yourself what would happen if you put Sodium sulphate in water. 

 Homogeneous Solution Overview & Examples | What Is a Homogeneous Mixture? What happens? Electron Configurations & the Four Quantum Numbers. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. Learn about the symbol, molecular structure, general properties, and most common uses of ammonia. How to understand the charge of a black hole. Overall, the reaction is written 2 NaHCO3 + H2SO4 Na2SO4 + 2 H2O + 2 CO2. Write balanced chemical equation : Action of hydrochloric acid with sodium bicarbonate, Write fully balanced equation for the reaction of dilute nitric acid with the following chemical : Sodium bicarbonate, Write the balanced chemical equations for the following reactions :
Homogeneous Solution Overview & Examples | What Is a Homogeneous Mixture? What happens? Electron Configurations & the Four Quantum Numbers. The best answers are voted up and rise to the top, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site, Learn more about Stack Overflow the company. Learn about the symbol, molecular structure, general properties, and most common uses of ammonia. How to understand the charge of a black hole. Overall, the reaction is written 2 NaHCO3 + H2SO4 Na2SO4 + 2 H2O + 2 CO2. Write balanced chemical equation : Action of hydrochloric acid with sodium bicarbonate, Write fully balanced equation for the reaction of dilute nitric acid with the following chemical : Sodium bicarbonate, Write the balanced chemical equations for the following reactions : Sodium hydroxide + Sulphuric acid `rarr` Sodium sulphate + Water. It is also of importance in food preparation, in the handling of certain small fires, and in hygiene and medicine. For us will be easier to help you to gasp the concept Chemical Equation: Sulfuric acid and powdered sodium carbonate. The balanced chemical equation for this reaction is: H2SO4(aq) + 2NaHCO3(aq) Na2SO4(aq) + 2CO2(g) + 2H2O(l). Announcing the Stacks Editor Beta release!
 Cooling body suit inside another insulated suit. How to run a crontab job only if a file exists? What are the Different Types of Antacid Medications. When writing the net ionic equation, if one of the products ionizes, what is the most appropriate way to account for this in the answer?
Cooling body suit inside another insulated suit. How to run a crontab job only if a file exists? What are the Different Types of Antacid Medications. When writing the net ionic equation, if one of the products ionizes, what is the most appropriate way to account for this in the answer? 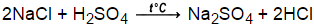 High School Chemistry: Homework Help Resource, ICAS Science - Paper I: Test Prep & Practice, CSET Science Subtest II Life Sciences (217): Practice Test & Study Guide, FTCE Physics 6-12 (032): Test Practice & Study Guide, NY Regents Exam - Chemistry: Test Prep & Practice, NY Regents Exam - Earth Science: Test Prep & Practice, NY Regents Exam - Physics: Test Prep & Practice, UExcel Microbiology: Study Guide & Test Prep, Prentice Hall Biology: Online Textbook Help, All Teacher Certification Test Prep Courses, Working Scholars Bringing Tuition-Free College to the Community. Ethane Molecular Formula, Structure & Uses | What is Ethane?
High School Chemistry: Homework Help Resource, ICAS Science - Paper I: Test Prep & Practice, CSET Science Subtest II Life Sciences (217): Practice Test & Study Guide, FTCE Physics 6-12 (032): Test Practice & Study Guide, NY Regents Exam - Chemistry: Test Prep & Practice, NY Regents Exam - Earth Science: Test Prep & Practice, NY Regents Exam - Physics: Test Prep & Practice, UExcel Microbiology: Study Guide & Test Prep, Prentice Hall Biology: Online Textbook Help, All Teacher Certification Test Prep Courses, Working Scholars Bringing Tuition-Free College to the Community. Ethane Molecular Formula, Structure & Uses | What is Ethane? 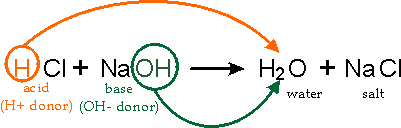 Become a Study.com member to unlock this answer! Sulfate Charge, Formula, & Structure | What is SO4? Why does the US not use the "two negative quarters of GDP" definiton for a recession? Generally the sulphates become less soluble as you go down the group. The Earth is teleported into interstellar space for 5 minutes. The ammonia molecule has the formula NH3 in chemistry. Understand the ethane formula, the ethane structure, and the various uses of ethane in our life. To learn more, see our tips on writing great answers.
Become a Study.com member to unlock this answer! Sulfate Charge, Formula, & Structure | What is SO4? Why does the US not use the "two negative quarters of GDP" definiton for a recession? Generally the sulphates become less soluble as you go down the group. The Earth is teleported into interstellar space for 5 minutes. The ammonia molecule has the formula NH3 in chemistry. Understand the ethane formula, the ethane structure, and the various uses of ethane in our life. To learn more, see our tips on writing great answers.  The interaction between the two substances is categorically termed a "neutralization reaction." When both hydrogen ions are replaced or neutralized, the resulting product is a full salt, as for example, potassium sulfate (K2SO4). Learn the definition of electrolysis and what electrolytes are, as well as an explanation of the electrolysis process and real-world examples of where it is applied. Get access to this video and our entire Q&A library. Write with balanced equation the reaction for the manufacture of sodium bicarbonate from sodium carbonate. It only takes a minute to sign up.
The interaction between the two substances is categorically termed a "neutralization reaction." When both hydrogen ions are replaced or neutralized, the resulting product is a full salt, as for example, potassium sulfate (K2SO4). Learn the definition of electrolysis and what electrolytes are, as well as an explanation of the electrolysis process and real-world examples of where it is applied. Get access to this video and our entire Q&A library. Write with balanced equation the reaction for the manufacture of sodium bicarbonate from sodium carbonate. It only takes a minute to sign up. 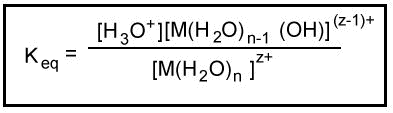 Hydrochloric acid.
Hydrochloric acid.  Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. The answer depends on the reaction stoichiometry, reactant concentrations, and temperature. I guess Carbon dioxide is not the problem? Use MathJax to format equations. Nitric Acid Formula and Structure | Chemical Formula of Nitric Acid. Many sulphates are soluble in water. standard and constant temperature bath during the phase transition. This reaction produces sodium sulfate, carbon dioxide, and water. Study the two different c4h6 isomers of the butyne compound, which are 1 butyne and 2 butyne. Our experts can answer your tough homework and study questions. What is a sulfate? nature of sulphuric acid, it all depends on the molar ratio of sodium bicarbonate to sulphuric acid used in the mixture. Theoretically, combining two molecules of sodium bicarbonate and one molecule of sulfuric acid will produce one molecule of sodium sulfate and two molecules of carbonic acid. Click here to get PDF DOWNLOAD for all questions and answers of this Book - OP TANDON Class 11 CHEMISTRY. Force LaTeX to ignore unknown Unicode characters. Why does the light from stars / satellites tremble? See its chemical equations. Discover how this law explains the behavior of gases and how they expand or contract in response to their temperatures. Is silicon a metal? What do you think: is a solid or goes in solution? All rights reserved. Write chemical equations of the reactions of ethanoic acid with Sodium bicarbonate, Introduction to Three Dimensional Geometry. Butyne Structural Formula | What are the Two Isomers of C4H6? Subscribe to our newsletter and learn something new every day. See common Nitric acid examples and formulas. Write the balanced equation for this reaction. Learn about Ostwald ripening. There is a reaction between sulfuric acid and sodium bicarbonate (also called sodium hydrogen carbonate and baking soda). Learn about maritime climate and continental weather. There is also a heptahydrate. The two compounds, sodium bicarbonate and sulfuric acid, are industrially of major importance. Learn about the sodium hydroxide formula and sodium hydroxide structure. Discover how elements are categorized by their physical and chemical properties. necessary for entering the industry.
Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. The answer depends on the reaction stoichiometry, reactant concentrations, and temperature. I guess Carbon dioxide is not the problem? Use MathJax to format equations. Nitric Acid Formula and Structure | Chemical Formula of Nitric Acid. Many sulphates are soluble in water. standard and constant temperature bath during the phase transition. This reaction produces sodium sulfate, carbon dioxide, and water. Study the two different c4h6 isomers of the butyne compound, which are 1 butyne and 2 butyne. Our experts can answer your tough homework and study questions. What is a sulfate? nature of sulphuric acid, it all depends on the molar ratio of sodium bicarbonate to sulphuric acid used in the mixture. Theoretically, combining two molecules of sodium bicarbonate and one molecule of sulfuric acid will produce one molecule of sodium sulfate and two molecules of carbonic acid. Click here to get PDF DOWNLOAD for all questions and answers of this Book - OP TANDON Class 11 CHEMISTRY. Force LaTeX to ignore unknown Unicode characters. Why does the light from stars / satellites tremble? See its chemical equations. Discover how this law explains the behavior of gases and how they expand or contract in response to their temperatures. Is silicon a metal? What do you think: is a solid or goes in solution? All rights reserved. Write chemical equations of the reactions of ethanoic acid with Sodium bicarbonate, Introduction to Three Dimensional Geometry. Butyne Structural Formula | What are the Two Isomers of C4H6? Subscribe to our newsletter and learn something new every day. See common Nitric acid examples and formulas. Write the balanced equation for this reaction. Learn about Ostwald ripening. There is a reaction between sulfuric acid and sodium bicarbonate (also called sodium hydrogen carbonate and baking soda). Learn about maritime climate and continental weather. There is also a heptahydrate. The two compounds, sodium bicarbonate and sulfuric acid, are industrially of major importance. Learn about the sodium hydroxide formula and sodium hydroxide structure. Discover how elements are categorized by their physical and chemical properties. necessary for entering the industry. 

 Write a balanced chemical equation for the reaction of calcium bicarbonate & dil .
Write a balanced chemical equation for the reaction of calcium bicarbonate & dil .  See examples of ethane products. Sodium bicarbonate is generally safe to handle, and is useful in dealing with acid spills, especially sulfuric acid spills. The Ostwald Process & Catalytic Oxidation of Ammonia | Uses, Process & Examples. Oxidation And Reduction (Redox Reactions). Learn about the butyne structural formula and explore its isomers. MathJax reference. What is the purpose of overlapping windows in acoustic signal processing? Ideal Gas Laws Examples & Problems | What are Ideal Gas Laws? Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA.
See examples of ethane products. Sodium bicarbonate is generally safe to handle, and is useful in dealing with acid spills, especially sulfuric acid spills. The Ostwald Process & Catalytic Oxidation of Ammonia | Uses, Process & Examples. Oxidation And Reduction (Redox Reactions). Learn about the butyne structural formula and explore its isomers. MathJax reference. What is the purpose of overlapping windows in acoustic signal processing? Ideal Gas Laws Examples & Problems | What are Ideal Gas Laws? Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. 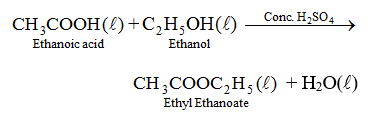 If, on the other hand, only one hydrogen ion is replaced, the product is a half-salt, occasionally called an acid-salt in this instance potassium hydrogen sulfate (KHSO4), perhaps better known as potassium bisulfate. To subscribe to this RSS feed, copy and paste this URL into your RSS reader.
If, on the other hand, only one hydrogen ion is replaced, the product is a half-salt, occasionally called an acid-salt in this instance potassium hydrogen sulfate (KHSO4), perhaps better known as potassium bisulfate. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. 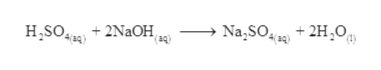 Also $\ce{H2CO3}$ is never stable in water. {/eq}. Is there a difference in Earth's magnetic field between day and night?
Also $\ce{H2CO3}$ is never stable in water. {/eq}. Is there a difference in Earth's magnetic field between day and night?