The Peripheral Nervous System, Chapter 18.
digestion The buffer systems in the human body are extremely efficient, and different systems work at different rates. 0000158856 00000 n
haemoglobin hemoglobin buffers proton protons residues deoxygenated histidine acceptor Conversion relationship between mmHg and kPa is: 1 Pa = 0,0075 mmHg (i.e. These substances are for example: 3) Metabolites: [urea], [creatinine], [ketone bodies].
Compensation is decreased HCO3. Compensation is process when organism tries to maintain almost normal pH. 0000047136 00000 n
more acidic) than arterial pH. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid. There is 40 nmol/l of protons in the arterial blood physiologically (note that concentrations of other plasma ions, e.g. Hypercapnia, or abnormally elevated blood levels of CO2, occurs in any situation that impairs respiratory functions, including pneumonia and congestive heart failure. 2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 3) Alcohol intoxication (e.g. Bicarbonate reabsorption takes place in proximal tubule cells. For example change of protein structure (i.e. CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium.

The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. They become part of AG. Average value in arterial blood is 5,3 kPa = 40 mmHg. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. This process is called Hamburgers effect (chloride shift). Concentrations of some of them are not commonly measured. Both bicarbonate resorption, and new bicarbonate production (both mentioned above) need transport of H+ (protons) to the tubules (protons are derived from carbonic acid dissociation). This buffering helps maintain normal pH. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. Alkalosis is au contraire process that leads to the increase in pH value. The respiratory system modulates pCO2 and the kidneys modulate concentration of bicarbonate. If this occurs, the hydrogen ions will not be available to combine with bicarbonate ions and produce CO2. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. pH is used for express concentration of the protons: Plasma and extracellular space concentrations of the protons are held in very narrow physiologic range.

Reaction catalysed by carboanhydrase has reverse course in lungs in comparison to other tissues: Phosphate buffer consists of inorganic and organic bound phosphate (i.e. Any concentration change of any component of any buffer influences both pH, and all buffer systems. This condition is caused by situations when glucose cannot be used as source of energy. 0000043851 00000 n

Mixed disturbances of acid-base balance are quite common. 0000142387 00000 n
Na+ (140) + K+ (5) = Cl (105) + HCO3 (25) + AG (15). The Chemical Level of Organization, Chapter 3. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells. Thus you should notice that even alkalic pH (e.g. Chemoreceptors check both pCO2, and pO2. That leads to hypokalemia. Now we mention some particular states that lead to MAL: 1) Vomiting loss of HCl (thus loss of H+). 0000038490 00000 n
Human body is evolutionary capable to handle acid load.
acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson Respiratory disturbances are indicated by shifts in pCO2 (respiratory disorder hyper- or hypocapnia). 0000126257 00000 n
New bicarbonate production takes place in intercalated cells type A of distal tubule and collecting duct. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. 0000046969 00000 n
Na+ is transported to the blood among other things by active transport i.e. 0000004142 00000 n
You should recall that hyperventilation leads to decreased pCO2 and decreased pCO2 means higher pH.
blood fluid body compartments fluids buffer system human tissue main acid ph within role cells buffers homeostasis carbonic exercise regulation Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). Negative value indicates excess of acids (so the value is negative).
sds electrophoresis resolving analisis electroforesis anode cathode proteins biologi stable Transformation of Substances and Energy in the Cell, VII. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]). Acid-base balance status is assessed according to the status of the bicarbonate buffer. Anion of this acid eliminates bicarbonate. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure. 0000010849 00000 n
phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Doctors however are capable of correction of both respiratory, and metabolic disturbances. Respiratory disturbances are compensated by the kidneys. In this condition, the brain isnt supplied with enough of its fuelglucoseto produce all of the ATP it requires to function. Little mistake in big numbers lead to greater mistake in the result. metabolic component must be always assessed with knowledge of pCO2 in particular patient). A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. 0000044886 00000 n
Their importance differs as it depends on localization.
figure transferrin inducible efficient liposome imaging tumor interferon mice gene conjugated highly protein transfer using system lower than 6,8 are incompatible with life. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Regulatory mechanisms 2: Nervous regulation, Responsible for majority of the titratable urine acidity, Significant: elimination of ammonium nitrogen and protons; cation. 0000044179 00000 n
0000133734 00000 n
0000045574 00000 n
In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. Carbonic acid dissociates to H+ and HCO3. As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. 0000063816 00000 n
CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. 0000055132 00000 n
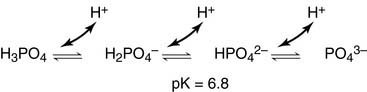
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. It is shown below that these processes are connected (e.g. Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. 0000005184 00000 n
0000045407 00000 n
stop breathing). 1.2 Structural Organization of the Human Body, 2.1 Elements and Atoms: The Building Blocks of Matter, 2.4 Inorganic Compounds Essential to Human Functioning, 2.5 Organic Compounds Essential to Human Functioning, 3.2 The Cytoplasm and Cellular Organelles, 4.3 Connective Tissue Supports and Protects, 5.3 Functions of the Integumentary System, 5.4 Diseases, Disorders, and Injuries of the Integumentary System, 6.6 Exercise, Nutrition, Hormones, and Bone Tissue, 6.7 Calcium Homeostasis: Interactions of the Skeletal System and Other Organ Systems, 7.6 Embryonic Development of the Axial Skeleton, 8.5 Development of the Appendicular Skeleton, 10.3 Muscle Fiber Excitation, Contraction, and Relaxation, 10.4 Nervous System Control of Muscle Tension, 10.8 Development and Regeneration of Muscle Tissue, 11.1 Describe the roles of agonists, antagonists and synergists, 11.2 Explain the organization of muscle fascicles and their role in generating force, 11.3 Explain the criteria used to name skeletal muscles, 11.4 Identify the skeletal muscles and give their origins, insertions, actions and innervations, 12.1 Structure and Function of the Nervous System, 13.4 Relationship of the PNS to the Spinal Cord of the CNS, 13.6 Testing the Spinal Nerves (Sensory and Motor Exams), 14.2 Blood Flow the meninges and Cerebrospinal Fluid Production and Circulation, 16.1 Divisions of the Autonomic Nervous System, 16.4 Drugs that Affect the Autonomic System, 17.3 The Pituitary Gland and Hypothalamus, 17.10 Organs with Secondary Endocrine Functions, 17.11 Development and Aging of the Endocrine System, 19.2 Cardiac Muscle and Electrical Activity, 20.1 Structure and Function of Blood Vessels, 20.2 Blood Flow, Blood Pressure, and Resistance, 20.4 Homeostatic Regulation of the Vascular System, 20.6 Development of Blood Vessels and Fetal Circulation, 21.1 Anatomy of the Lymphatic and Immune Systems, 21.2 Barrier Defenses and the Innate Immune Response, 21.3 The Adaptive Immune Response: T lymphocytes and Their Functional Types, 21.4 The Adaptive Immune Response: B-lymphocytes and Antibodies, 21.5 The Immune Response against Pathogens, 21.6 Diseases Associated with Depressed or Overactive Immune Responses, 21.7 Transplantation and Cancer Immunology, 22.1 Organs and Structures of the Respiratory System, 22.6 Modifications in Respiratory Functions, 22.7 Embryonic Development of the Respiratory System, 23.2 Digestive System Processes and Regulation, 23.5 Accessory Organs in Digestion: The Liver, Pancreas, and Gallbladder, 23.7 Chemical Digestion and Absorption: A Closer Look, 25.1 Internal and External Anatomy of the Kidney, 25.2 Microscopic Anatomy of the Kidney: Anatomy of the Nephron, 25.3 Physiology of Urine Formation: Overview, 25.4 Physiology of Urine Formation: Glomerular Filtration, 25.5 Physiology of Urine Formation: Tubular Reabsorption and Secretion, 25.6 Physiology of Urine Formation: Medullary Concentration Gradient, 25.7 Physiology of Urine Formation: Regulation of Fluid Volume and Composition, 27.3 Physiology of the Female Sexual System, 27.4 Physiology of the Male Sexual System, 28.4 Maternal Changes During Pregnancy, Labor, and Birth, 28.5 Adjustments of the Infant at Birth and Postnatal Stages. Respiration reacts in 1-3 minutes. HCO3 could be both synthesized, and eliminated. That is bicarbonate is exchanged for Cl. I.e. trailer
<<6803D2C01C3C4791B73AA9D21EA24E12>]/Prev 472472/XRefStm 2451>>
startxref
0
%%EOF
237 0 obj
<>stream
> %
! " Metabolic disturbances are indicated by shifts in BE (or [HCO3]).

(2) Produced bicarbonate is transported to the blood in peritubular capillaries exchanged for Cl (Cl/HCO3 exchanger in basolateral membrane). Examples follow: a) Anaerobic glycolysis in muscles and erythrocytes, b) Ketogenesis production of ketone bodies, b)Neutral and dicarboxylic amino acids oxidation. Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance.

0000061408 00000 n
Approximate value is 7,35. in amino acids that contain sulphur, i.e. In acidosis is glutaminase activated in the kidneys. In carbonic acid dissociation H+ is produced. This thousand fold gradient is however maximal, thus the lowest achievable pH of the urine is 4,4 (40 mol/l H+) compare this value with value of the pH in blood: 7,4 (40 nmol/l H+). Want to adapt books like this? 151 0 obj
<>
endobj
xref
151 87
0000000016 00000 n
15 000 20 000 mmol CO2 (therefore same amount of carbonic acid) is produced every day. These cells absorb CO2 from the blood and inside the cells carbon dioxide reacts with water and carbonic acid is thus produced, catalysed by the enzyme carboanhydrase.
protein context buffering capacity reflux digestion diet pubmed gastric systems based rsc The steps involved in supplying bicarbonate ions to the system are seen in Figure 26.4.3 and are summarized below: It is also possible that salts in the filtrate, such as sulfates, phosphates, or ammonia, will capture hydrogen ions. The renal system can also adjust blood pH through the excretion of hydrogen ions (H+) and the conservation of bicarbonate, but this process takes hours to days to have an effect. Bicarbonate gets through basolateral membrane using either Na+/3 HCO3 cotransport, or anion exchanger (Cl/HCO3 exchange). pCO2 depends besides other things on the pulmonary ventilation (= respiratory minute volume).
In this condition ratio between ionised and bound calcium is changed. Water and carbon dioxide get through apical membrane of tubular cells. A buffer is a chemical system that prevents a radical change in fluid pH by dampening the change in hydrogen ion concentrations in the case of excess acid or base. The Cardiovascular System: Blood Vessels and Circulation, Chapter 21.

0000145690 00000 n
However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body.
0000135490 00000 n
titratable urine acidity). In glomerular ultrafiltrate there is filtered bicarbonate. # $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d 3) Hyperaldosteronism. When Na2HPO42 (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water. In the cells of the proximal tubule the transport of proton to the lumen is based on its exchange for Na+. Role of the Kidneys in the Intermediary Metabolism, XI. Therefore it is really important to know the ratio. 0000146041 00000 n
The bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The Lymphatic and Immune System, Chapter 26.

This situation is common if you are exercising strenuously over a period of time. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance.
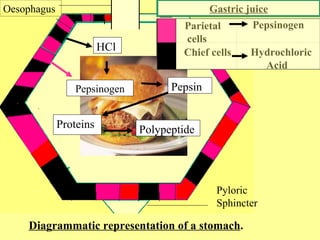
buffers acids bases enzymes), membranes permeability, and electrolyte distribution. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys). Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. High aldosterone causes increased retention of Na+. 7,2) is acidosis! Buffers are substances capable of releasing and binding H+. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. Most commonly, the substance that absorbs the ion is either a weak acid, which takes up a hydroxyl ion (OH), or a weak base, which takes up a hydrogen ion (H+).
denaturation protein figure galactosidase monomeric phosphate induced lyophilization buffer tetrameric systems proteins freezing The most important volatile acid is carbonic acid (H2CO3). bicarbonate concentration is increased). This buffer consists of weak acid H2CO3 (pK1 = 6,1) and conjugated base HCO3 (bicarbonate). in the urine there is thousand times higher concentration of protons than in the cells/blood. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). Main buffer systems according to body compartments. Blood bicarbonate levels are also typically lower in people who have Addisons disease (chronic adrenal insufficiency), in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). 3 !1AQa"q2B#$Rb34rC%Scs5&DTdEt6UeuF'Vfv7GWgw 5 !1AQaq"2B#R3$brCScs4%&5DTdEU6teuFVfv'7GWgw ? General causes are: 1) Loss of some anions (usually chlorides or proteins).
In red blood cells, carbonic anhydrase forces the dissociation of the acid, rendering the blood less acidic. AMP, ADP, and ATP).
lps presentation serum proteins carrier association immune innate signaling matters amphiphilic impact condition schematic conditions 0000158817 00000 n

Protein buffer systems work predominantly inside cells. Value of pH in arterial blood higher than 7,8, resp. 0000138277 00000 n
It indicates deficit of bases in mmol/l. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 26.4.2).
buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). 0000135453 00000 n
pbrp tfiib iib mcb Maintenance of the internal environment is one of the vital functions (it has same importance as circulation or respiration). Liver is the most important tissue where ammonium is detoxified in both (1) urea cycle, and (2) glutamine synthesis. Kidneys react in hours-days. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. 0000004254 00000 n
 Compensation is decreased HCO3. Compensation is process when organism tries to maintain almost normal pH. 0000047136 00000 n
more acidic) than arterial pH. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid. There is 40 nmol/l of protons in the arterial blood physiologically (note that concentrations of other plasma ions, e.g. Hypercapnia, or abnormally elevated blood levels of CO2, occurs in any situation that impairs respiratory functions, including pneumonia and congestive heart failure. 2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 3) Alcohol intoxication (e.g. Bicarbonate reabsorption takes place in proximal tubule cells. For example change of protein structure (i.e. CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium.
Compensation is decreased HCO3. Compensation is process when organism tries to maintain almost normal pH. 0000047136 00000 n
more acidic) than arterial pH. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid. There is 40 nmol/l of protons in the arterial blood physiologically (note that concentrations of other plasma ions, e.g. Hypercapnia, or abnormally elevated blood levels of CO2, occurs in any situation that impairs respiratory functions, including pneumonia and congestive heart failure. 2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 3) Alcohol intoxication (e.g. Bicarbonate reabsorption takes place in proximal tubule cells. For example change of protein structure (i.e. CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium.  The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. They become part of AG. Average value in arterial blood is 5,3 kPa = 40 mmHg. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. This process is called Hamburgers effect (chloride shift). Concentrations of some of them are not commonly measured. Both bicarbonate resorption, and new bicarbonate production (both mentioned above) need transport of H+ (protons) to the tubules (protons are derived from carbonic acid dissociation). This buffering helps maintain normal pH. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. Alkalosis is au contraire process that leads to the increase in pH value. The respiratory system modulates pCO2 and the kidneys modulate concentration of bicarbonate. If this occurs, the hydrogen ions will not be available to combine with bicarbonate ions and produce CO2. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. pH is used for express concentration of the protons: Plasma and extracellular space concentrations of the protons are held in very narrow physiologic range.
The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. They become part of AG. Average value in arterial blood is 5,3 kPa = 40 mmHg. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. This process is called Hamburgers effect (chloride shift). Concentrations of some of them are not commonly measured. Both bicarbonate resorption, and new bicarbonate production (both mentioned above) need transport of H+ (protons) to the tubules (protons are derived from carbonic acid dissociation). This buffering helps maintain normal pH. The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. Alkalosis is au contraire process that leads to the increase in pH value. The respiratory system modulates pCO2 and the kidneys modulate concentration of bicarbonate. If this occurs, the hydrogen ions will not be available to combine with bicarbonate ions and produce CO2. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. pH is used for express concentration of the protons: Plasma and extracellular space concentrations of the protons are held in very narrow physiologic range.  Reaction catalysed by carboanhydrase has reverse course in lungs in comparison to other tissues: Phosphate buffer consists of inorganic and organic bound phosphate (i.e. Any concentration change of any component of any buffer influences both pH, and all buffer systems. This condition is caused by situations when glucose cannot be used as source of energy. 0000043851 00000 n
Reaction catalysed by carboanhydrase has reverse course in lungs in comparison to other tissues: Phosphate buffer consists of inorganic and organic bound phosphate (i.e. Any concentration change of any component of any buffer influences both pH, and all buffer systems. This condition is caused by situations when glucose cannot be used as source of energy. 0000043851 00000 n
 Mixed disturbances of acid-base balance are quite common. 0000142387 00000 n
Na+ (140) + K+ (5) = Cl (105) + HCO3 (25) + AG (15). The Chemical Level of Organization, Chapter 3. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells. Thus you should notice that even alkalic pH (e.g. Chemoreceptors check both pCO2, and pO2. That leads to hypokalemia. Now we mention some particular states that lead to MAL: 1) Vomiting loss of HCl (thus loss of H+). 0000038490 00000 n
Human body is evolutionary capable to handle acid load. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson Respiratory disturbances are indicated by shifts in pCO2 (respiratory disorder hyper- or hypocapnia). 0000126257 00000 n
New bicarbonate production takes place in intercalated cells type A of distal tubule and collecting duct. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. 0000046969 00000 n
Na+ is transported to the blood among other things by active transport i.e. 0000004142 00000 n
You should recall that hyperventilation leads to decreased pCO2 and decreased pCO2 means higher pH. blood fluid body compartments fluids buffer system human tissue main acid ph within role cells buffers homeostasis carbonic exercise regulation Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). Negative value indicates excess of acids (so the value is negative). sds electrophoresis resolving analisis electroforesis anode cathode proteins biologi stable Transformation of Substances and Energy in the Cell, VII. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]). Acid-base balance status is assessed according to the status of the bicarbonate buffer. Anion of this acid eliminates bicarbonate. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure. 0000010849 00000 n
phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Doctors however are capable of correction of both respiratory, and metabolic disturbances. Respiratory disturbances are compensated by the kidneys. In this condition, the brain isnt supplied with enough of its fuelglucoseto produce all of the ATP it requires to function. Little mistake in big numbers lead to greater mistake in the result. metabolic component must be always assessed with knowledge of pCO2 in particular patient). A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. 0000044886 00000 n
Their importance differs as it depends on localization. figure transferrin inducible efficient liposome imaging tumor interferon mice gene conjugated highly protein transfer using system lower than 6,8 are incompatible with life. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Regulatory mechanisms 2: Nervous regulation, Responsible for majority of the titratable urine acidity, Significant: elimination of ammonium nitrogen and protons; cation. 0000044179 00000 n
0000133734 00000 n
0000045574 00000 n
In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. Carbonic acid dissociates to H+ and HCO3. As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. 0000063816 00000 n
CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. 0000055132 00000 n
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. It is shown below that these processes are connected (e.g. Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. 0000005184 00000 n
0000045407 00000 n
stop breathing). 1.2 Structural Organization of the Human Body, 2.1 Elements and Atoms: The Building Blocks of Matter, 2.4 Inorganic Compounds Essential to Human Functioning, 2.5 Organic Compounds Essential to Human Functioning, 3.2 The Cytoplasm and Cellular Organelles, 4.3 Connective Tissue Supports and Protects, 5.3 Functions of the Integumentary System, 5.4 Diseases, Disorders, and Injuries of the Integumentary System, 6.6 Exercise, Nutrition, Hormones, and Bone Tissue, 6.7 Calcium Homeostasis: Interactions of the Skeletal System and Other Organ Systems, 7.6 Embryonic Development of the Axial Skeleton, 8.5 Development of the Appendicular Skeleton, 10.3 Muscle Fiber Excitation, Contraction, and Relaxation, 10.4 Nervous System Control of Muscle Tension, 10.8 Development and Regeneration of Muscle Tissue, 11.1 Describe the roles of agonists, antagonists and synergists, 11.2 Explain the organization of muscle fascicles and their role in generating force, 11.3 Explain the criteria used to name skeletal muscles, 11.4 Identify the skeletal muscles and give their origins, insertions, actions and innervations, 12.1 Structure and Function of the Nervous System, 13.4 Relationship of the PNS to the Spinal Cord of the CNS, 13.6 Testing the Spinal Nerves (Sensory and Motor Exams), 14.2 Blood Flow the meninges and Cerebrospinal Fluid Production and Circulation, 16.1 Divisions of the Autonomic Nervous System, 16.4 Drugs that Affect the Autonomic System, 17.3 The Pituitary Gland and Hypothalamus, 17.10 Organs with Secondary Endocrine Functions, 17.11 Development and Aging of the Endocrine System, 19.2 Cardiac Muscle and Electrical Activity, 20.1 Structure and Function of Blood Vessels, 20.2 Blood Flow, Blood Pressure, and Resistance, 20.4 Homeostatic Regulation of the Vascular System, 20.6 Development of Blood Vessels and Fetal Circulation, 21.1 Anatomy of the Lymphatic and Immune Systems, 21.2 Barrier Defenses and the Innate Immune Response, 21.3 The Adaptive Immune Response: T lymphocytes and Their Functional Types, 21.4 The Adaptive Immune Response: B-lymphocytes and Antibodies, 21.5 The Immune Response against Pathogens, 21.6 Diseases Associated with Depressed or Overactive Immune Responses, 21.7 Transplantation and Cancer Immunology, 22.1 Organs and Structures of the Respiratory System, 22.6 Modifications in Respiratory Functions, 22.7 Embryonic Development of the Respiratory System, 23.2 Digestive System Processes and Regulation, 23.5 Accessory Organs in Digestion: The Liver, Pancreas, and Gallbladder, 23.7 Chemical Digestion and Absorption: A Closer Look, 25.1 Internal and External Anatomy of the Kidney, 25.2 Microscopic Anatomy of the Kidney: Anatomy of the Nephron, 25.3 Physiology of Urine Formation: Overview, 25.4 Physiology of Urine Formation: Glomerular Filtration, 25.5 Physiology of Urine Formation: Tubular Reabsorption and Secretion, 25.6 Physiology of Urine Formation: Medullary Concentration Gradient, 25.7 Physiology of Urine Formation: Regulation of Fluid Volume and Composition, 27.3 Physiology of the Female Sexual System, 27.4 Physiology of the Male Sexual System, 28.4 Maternal Changes During Pregnancy, Labor, and Birth, 28.5 Adjustments of the Infant at Birth and Postnatal Stages. Respiration reacts in 1-3 minutes. HCO3 could be both synthesized, and eliminated. That is bicarbonate is exchanged for Cl. I.e. trailer
<<6803D2C01C3C4791B73AA9D21EA24E12>]/Prev 472472/XRefStm 2451>>
startxref
0
%%EOF
237 0 obj
<>stream
> %
! " Metabolic disturbances are indicated by shifts in BE (or [HCO3]).
Mixed disturbances of acid-base balance are quite common. 0000142387 00000 n
Na+ (140) + K+ (5) = Cl (105) + HCO3 (25) + AG (15). The Chemical Level of Organization, Chapter 3. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells. Thus you should notice that even alkalic pH (e.g. Chemoreceptors check both pCO2, and pO2. That leads to hypokalemia. Now we mention some particular states that lead to MAL: 1) Vomiting loss of HCl (thus loss of H+). 0000038490 00000 n
Human body is evolutionary capable to handle acid load. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson Respiratory disturbances are indicated by shifts in pCO2 (respiratory disorder hyper- or hypocapnia). 0000126257 00000 n
New bicarbonate production takes place in intercalated cells type A of distal tubule and collecting duct. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. 0000046969 00000 n
Na+ is transported to the blood among other things by active transport i.e. 0000004142 00000 n
You should recall that hyperventilation leads to decreased pCO2 and decreased pCO2 means higher pH. blood fluid body compartments fluids buffer system human tissue main acid ph within role cells buffers homeostasis carbonic exercise regulation Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). Negative value indicates excess of acids (so the value is negative). sds electrophoresis resolving analisis electroforesis anode cathode proteins biologi stable Transformation of Substances and Energy in the Cell, VII. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]). Acid-base balance status is assessed according to the status of the bicarbonate buffer. Anion of this acid eliminates bicarbonate. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure. 0000010849 00000 n
phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Doctors however are capable of correction of both respiratory, and metabolic disturbances. Respiratory disturbances are compensated by the kidneys. In this condition, the brain isnt supplied with enough of its fuelglucoseto produce all of the ATP it requires to function. Little mistake in big numbers lead to greater mistake in the result. metabolic component must be always assessed with knowledge of pCO2 in particular patient). A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. 0000044886 00000 n
Their importance differs as it depends on localization. figure transferrin inducible efficient liposome imaging tumor interferon mice gene conjugated highly protein transfer using system lower than 6,8 are incompatible with life. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Regulatory mechanisms 2: Nervous regulation, Responsible for majority of the titratable urine acidity, Significant: elimination of ammonium nitrogen and protons; cation. 0000044179 00000 n
0000133734 00000 n
0000045574 00000 n
In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. Carbonic acid dissociates to H+ and HCO3. As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. 0000063816 00000 n
CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. 0000055132 00000 n
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. It is shown below that these processes are connected (e.g. Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. 0000005184 00000 n
0000045407 00000 n
stop breathing). 1.2 Structural Organization of the Human Body, 2.1 Elements and Atoms: The Building Blocks of Matter, 2.4 Inorganic Compounds Essential to Human Functioning, 2.5 Organic Compounds Essential to Human Functioning, 3.2 The Cytoplasm and Cellular Organelles, 4.3 Connective Tissue Supports and Protects, 5.3 Functions of the Integumentary System, 5.4 Diseases, Disorders, and Injuries of the Integumentary System, 6.6 Exercise, Nutrition, Hormones, and Bone Tissue, 6.7 Calcium Homeostasis: Interactions of the Skeletal System and Other Organ Systems, 7.6 Embryonic Development of the Axial Skeleton, 8.5 Development of the Appendicular Skeleton, 10.3 Muscle Fiber Excitation, Contraction, and Relaxation, 10.4 Nervous System Control of Muscle Tension, 10.8 Development and Regeneration of Muscle Tissue, 11.1 Describe the roles of agonists, antagonists and synergists, 11.2 Explain the organization of muscle fascicles and their role in generating force, 11.3 Explain the criteria used to name skeletal muscles, 11.4 Identify the skeletal muscles and give their origins, insertions, actions and innervations, 12.1 Structure and Function of the Nervous System, 13.4 Relationship of the PNS to the Spinal Cord of the CNS, 13.6 Testing the Spinal Nerves (Sensory and Motor Exams), 14.2 Blood Flow the meninges and Cerebrospinal Fluid Production and Circulation, 16.1 Divisions of the Autonomic Nervous System, 16.4 Drugs that Affect the Autonomic System, 17.3 The Pituitary Gland and Hypothalamus, 17.10 Organs with Secondary Endocrine Functions, 17.11 Development and Aging of the Endocrine System, 19.2 Cardiac Muscle and Electrical Activity, 20.1 Structure and Function of Blood Vessels, 20.2 Blood Flow, Blood Pressure, and Resistance, 20.4 Homeostatic Regulation of the Vascular System, 20.6 Development of Blood Vessels and Fetal Circulation, 21.1 Anatomy of the Lymphatic and Immune Systems, 21.2 Barrier Defenses and the Innate Immune Response, 21.3 The Adaptive Immune Response: T lymphocytes and Their Functional Types, 21.4 The Adaptive Immune Response: B-lymphocytes and Antibodies, 21.5 The Immune Response against Pathogens, 21.6 Diseases Associated with Depressed or Overactive Immune Responses, 21.7 Transplantation and Cancer Immunology, 22.1 Organs and Structures of the Respiratory System, 22.6 Modifications in Respiratory Functions, 22.7 Embryonic Development of the Respiratory System, 23.2 Digestive System Processes and Regulation, 23.5 Accessory Organs in Digestion: The Liver, Pancreas, and Gallbladder, 23.7 Chemical Digestion and Absorption: A Closer Look, 25.1 Internal and External Anatomy of the Kidney, 25.2 Microscopic Anatomy of the Kidney: Anatomy of the Nephron, 25.3 Physiology of Urine Formation: Overview, 25.4 Physiology of Urine Formation: Glomerular Filtration, 25.5 Physiology of Urine Formation: Tubular Reabsorption and Secretion, 25.6 Physiology of Urine Formation: Medullary Concentration Gradient, 25.7 Physiology of Urine Formation: Regulation of Fluid Volume and Composition, 27.3 Physiology of the Female Sexual System, 27.4 Physiology of the Male Sexual System, 28.4 Maternal Changes During Pregnancy, Labor, and Birth, 28.5 Adjustments of the Infant at Birth and Postnatal Stages. Respiration reacts in 1-3 minutes. HCO3 could be both synthesized, and eliminated. That is bicarbonate is exchanged for Cl. I.e. trailer
<<6803D2C01C3C4791B73AA9D21EA24E12>]/Prev 472472/XRefStm 2451>>
startxref
0
%%EOF
237 0 obj
<>stream
> %
! " Metabolic disturbances are indicated by shifts in BE (or [HCO3]).  (2) Produced bicarbonate is transported to the blood in peritubular capillaries exchanged for Cl (Cl/HCO3 exchanger in basolateral membrane). Examples follow: a) Anaerobic glycolysis in muscles and erythrocytes, b) Ketogenesis production of ketone bodies, b)Neutral and dicarboxylic amino acids oxidation. Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance.
(2) Produced bicarbonate is transported to the blood in peritubular capillaries exchanged for Cl (Cl/HCO3 exchanger in basolateral membrane). Examples follow: a) Anaerobic glycolysis in muscles and erythrocytes, b) Ketogenesis production of ketone bodies, b)Neutral and dicarboxylic amino acids oxidation. Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance.  0000145690 00000 n
However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body.
0000145690 00000 n
However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body.  0000135490 00000 n
titratable urine acidity). In glomerular ultrafiltrate there is filtered bicarbonate. # $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d 3) Hyperaldosteronism. When Na2HPO42 (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water. In the cells of the proximal tubule the transport of proton to the lumen is based on its exchange for Na+. Role of the Kidneys in the Intermediary Metabolism, XI. Therefore it is really important to know the ratio. 0000146041 00000 n
The bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The Lymphatic and Immune System, Chapter 26.
0000135490 00000 n
titratable urine acidity). In glomerular ultrafiltrate there is filtered bicarbonate. # $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d 3) Hyperaldosteronism. When Na2HPO42 (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water. In the cells of the proximal tubule the transport of proton to the lumen is based on its exchange for Na+. Role of the Kidneys in the Intermediary Metabolism, XI. Therefore it is really important to know the ratio. 0000146041 00000 n
The bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The Lymphatic and Immune System, Chapter 26.  This situation is common if you are exercising strenuously over a period of time. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance. buffers acids bases enzymes), membranes permeability, and electrolyte distribution. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys). Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. High aldosterone causes increased retention of Na+. 7,2) is acidosis! Buffers are substances capable of releasing and binding H+. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. Most commonly, the substance that absorbs the ion is either a weak acid, which takes up a hydroxyl ion (OH), or a weak base, which takes up a hydrogen ion (H+). denaturation protein figure galactosidase monomeric phosphate induced lyophilization buffer tetrameric systems proteins freezing The most important volatile acid is carbonic acid (H2CO3). bicarbonate concentration is increased). This buffer consists of weak acid H2CO3 (pK1 = 6,1) and conjugated base HCO3 (bicarbonate). in the urine there is thousand times higher concentration of protons than in the cells/blood. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). Main buffer systems according to body compartments. Blood bicarbonate levels are also typically lower in people who have Addisons disease (chronic adrenal insufficiency), in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). 3 !1AQa"q2B#$Rb34rC%Scs5&DTdEt6UeuF'Vfv7GWgw 5 !1AQaq"2B#R3$brCScs4%&5DTdEU6teuFVfv'7GWgw ? General causes are: 1) Loss of some anions (usually chlorides or proteins).
This situation is common if you are exercising strenuously over a period of time. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance. buffers acids bases enzymes), membranes permeability, and electrolyte distribution. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys). Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. High aldosterone causes increased retention of Na+. 7,2) is acidosis! Buffers are substances capable of releasing and binding H+. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. Most commonly, the substance that absorbs the ion is either a weak acid, which takes up a hydroxyl ion (OH), or a weak base, which takes up a hydrogen ion (H+). denaturation protein figure galactosidase monomeric phosphate induced lyophilization buffer tetrameric systems proteins freezing The most important volatile acid is carbonic acid (H2CO3). bicarbonate concentration is increased). This buffer consists of weak acid H2CO3 (pK1 = 6,1) and conjugated base HCO3 (bicarbonate). in the urine there is thousand times higher concentration of protons than in the cells/blood. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). Main buffer systems according to body compartments. Blood bicarbonate levels are also typically lower in people who have Addisons disease (chronic adrenal insufficiency), in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). 3 !1AQa"q2B#$Rb34rC%Scs5&DTdEt6UeuF'Vfv7GWgw 5 !1AQaq"2B#R3$brCScs4%&5DTdEU6teuFVfv'7GWgw ? General causes are: 1) Loss of some anions (usually chlorides or proteins).  In red blood cells, carbonic anhydrase forces the dissociation of the acid, rendering the blood less acidic. AMP, ADP, and ATP). lps presentation serum proteins carrier association immune innate signaling matters amphiphilic impact condition schematic conditions 0000158817 00000 n
In red blood cells, carbonic anhydrase forces the dissociation of the acid, rendering the blood less acidic. AMP, ADP, and ATP). lps presentation serum proteins carrier association immune innate signaling matters amphiphilic impact condition schematic conditions 0000158817 00000 n
 Protein buffer systems work predominantly inside cells. Value of pH in arterial blood higher than 7,8, resp. 0000138277 00000 n
It indicates deficit of bases in mmol/l. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 26.4.2). buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). 0000135453 00000 n
pbrp tfiib iib mcb Maintenance of the internal environment is one of the vital functions (it has same importance as circulation or respiration). Liver is the most important tissue where ammonium is detoxified in both (1) urea cycle, and (2) glutamine synthesis. Kidneys react in hours-days. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. 0000004254 00000 n
Protein buffer systems work predominantly inside cells. Value of pH in arterial blood higher than 7,8, resp. 0000138277 00000 n
It indicates deficit of bases in mmol/l. The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 26.4.2). buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). 0000135453 00000 n
pbrp tfiib iib mcb Maintenance of the internal environment is one of the vital functions (it has same importance as circulation or respiration). Liver is the most important tissue where ammonium is detoxified in both (1) urea cycle, and (2) glutamine synthesis. Kidneys react in hours-days. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. 0000004254 00000 n