metals metal pure metalloids titanium chemistry atomic hafnium hf number transition Indium is a post-transition metal that makes up 0.21 parts per million of the Earths crust. Which of the following is a characteristic of the modern periodic table? Chemically similar to tin, germanium is an important semiconductor.

December 31, 2021 by Admin.

The mean pressure over the indentation is
Germanium - Minerals Education Coalition The germanium(ii) center of dicationic compound 1 donates an electron pair to transition metal Ag(i) and Au(i) cations, leading to complexes 2 and 3 respectively.
Wikipedia Germanium | Encyclopedia.com Hydrogen from formic acid through its selective transition metal, any of various chemical elements that have valence electronsi.e., electrons that can participate in the formation of chemical bondsin two shells instead of only one. In chemistry, the term transition metal (or transition element) has three possible definitions: . A HARD indenter pressed into the surface of a ductile solid such as a metal will produce a permanent indentation. These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It's hard at room temperature and looks metallic with a shiny silvery grey finish, but it's a semiconductor, without some of the key properties of a metal. Sometimes zinc, cadmium, and mercury are categorized with the post-transition metals rather than the transition metals. Germaniums refraction and dispersion properties make it useful in wide-angle camera lenses and in microscope objective lenses. Metalloids have properties of both metals and non-metals. Transition metal impurities in germanium introduce deep levels in the band gap, which may influence the lifetime of carriers and leakage currents of devices. indeed, previous studies of singly transition metal doped germanium clusters showed that starting from the size n = 9, the ge unit absorbs the ni and ru dopant endohedrally in giving rise to the most stable isomers of ge n ni and ge n ru. semiconductor. In its pure state, the element is crystalline and brittle, retaining its luster in air at room temperature. . Germanium is a chemical element with element symbol Ge, atomic number 32 and atomic weight 72.64. It belongs to carbon group. The surface preparation primarily using in-situ H 2 plasma
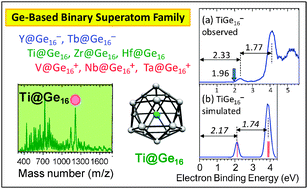 Germanium - MMTA Germaniumtransition-metal bonds: the importance of the leaving germanium
Germanium - MMTA Germaniumtransition-metal bonds: the importance of the leaving germanium it is non toxic in nature .
Germanium The large separation between the metal centers and the unhindered access of reactants to these active sites through uniformly sized channels make these materials a good point of departure for designing new catalysts.
germanium oxide chloride aldrich ftir metals basis crystalline ereztech geo2 sigmaaldrich dioxide raman K). Synthesis of Transition Metal Germanium Triple Bond Germylyne Complexes. According to the latest definition, germanium is considered a semiconductor.
Transition Metal Germanium is a chemical element with symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in thecarbon group, chemically similar to its group neighbors tin and silicon. Germanium is a gray white quasi metal, shiny and hard. Germanium is a metalloid. A metalloid is an element that has characteristics of both metals and non-metals. Germanium is located in the middle of the carbon family, which is Group 14 (IVA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. other reactions that have been shown to generate germanium-centred radicals include hydrogen atom transfer from organometallic hydrides to electronically excited ion transition metal complexes (equation (234) ), 280 photochemical decomposition of 7-germanorbornadienes ( scheme 61 ), 66,281 thermal- and photoinduced homolytic cleavage of ge ge Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Metal Digermyl, Germylsilyl and Germylstannyl Complexes.
Germanium: Definition & Uses | Study.com The post-transition metals, also known as the poor metals, is a group of metals on the periodic table. These species have been characterized by a variety of spectroscopic techniques and in the case of 3,4-dimet It is located in the 4th cycle and IVA group in the periodic table of chemical elements. It show hard, brittle , lustrous metal like properties.

E) gas. Germanium can be separated from other metals by fractional distillation of its volatile tetrachloride.

Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more Interesting Facts about Post-transition Metals. Noble gases are the group 18 elements (that have completed electron configurations). 2008-12-18 19:55:48.
transition metal 4. A link is provided below for more information.
germanium 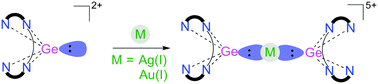
Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Indium. As long as there is a signal output logic 0 in A1 ~ An, Output is logic 0. Germanium is a gray white quasi metal, shiny and hard.
Germanium These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It also is a semiconductor, which means it has electrical conductivity between an insulator and a conductor.
germanium ligands chemistryviews Germanium is metalloid, which means it has properties of both metals and nonmetals. It is a brittle and stable element, being unaffected by air or water, acids or alkalis. It represent Metalloids have properties of both metals and non-metals.
germanium Thin film reaction of transition metals with germanium STEEP-SLOPE FIELD-EFFECT TRANSISTOR AND FABRICATION Answer: (a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal. As shown in the figure below, n Schottky diodes form an n-input AND gate. Tin. The element is a gray-white metalloid. It belongs to carbon group.
Metals in germanium Periodic Table Is germanium a metal nonmetal or The metal-germanium alloy layer and the Figure 7. In contrast the transition metal silicon or germanium bonds in the octahedral complexes of manganese, rhenium and tungsten are always cleaved with poor retention of configuration regardless of the nature of the ligands or the nucleophilic reagent. Among the several strateg Spotlight Collection: Lanthanide and transition metal We report the first example of GeF activation using a transition metal complex, in which -bond metathesis between GeF and IrH -bonds takes place with a specific electron flow.
transition metal | Definition, Properties, Elements, & Facts Is Lead Transition Metal - Classification - BYJUS is a transition metal Metals in germanium | Request PDF - ResearchGate The Group 12 elements are sometimes included.
bohr atomic energy germanium ge structure level atom number diagram elements levels Argon is in group 8A (18), and bromine is in group 7A (17). metal nonmetal metalloid main-group (representative) element transition metal inner transition metal Classify cesium as either a metal or nonmetal. A new zeolitetype structure is adopted by (NH4)+(M(NH3)2)+(Ge9O19)2 (M=Cu, Ag; shown in the picture). This is the first such catalyst not based on transition metals, and it exhibits very encouraging performance.
Indentation hardness and semiconductormetal transition of germanium 
Named for the country of Germany, germanium is not considered a metal, despite its metallic grayish-white appearance. They are to the right of the transition metals.
 Germanium in Periodic Table | Net Explanations germanium
Germanium in Periodic Table | Net Explanations germanium
 December 31, 2021 by Admin.
December 31, 2021 by Admin.  The mean pressure over the indentation is Germanium - Minerals Education Coalition The germanium(ii) center of dicationic compound 1 donates an electron pair to transition metal Ag(i) and Au(i) cations, leading to complexes 2 and 3 respectively. Wikipedia Germanium | Encyclopedia.com Hydrogen from formic acid through its selective transition metal, any of various chemical elements that have valence electronsi.e., electrons that can participate in the formation of chemical bondsin two shells instead of only one. In chemistry, the term transition metal (or transition element) has three possible definitions: . A HARD indenter pressed into the surface of a ductile solid such as a metal will produce a permanent indentation. These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It's hard at room temperature and looks metallic with a shiny silvery grey finish, but it's a semiconductor, without some of the key properties of a metal. Sometimes zinc, cadmium, and mercury are categorized with the post-transition metals rather than the transition metals. Germaniums refraction and dispersion properties make it useful in wide-angle camera lenses and in microscope objective lenses. Metalloids have properties of both metals and non-metals. Transition metal impurities in germanium introduce deep levels in the band gap, which may influence the lifetime of carriers and leakage currents of devices. indeed, previous studies of singly transition metal doped germanium clusters showed that starting from the size n = 9, the ge unit absorbs the ni and ru dopant endohedrally in giving rise to the most stable isomers of ge n ni and ge n ru. semiconductor. In its pure state, the element is crystalline and brittle, retaining its luster in air at room temperature. . Germanium is a chemical element with element symbol Ge, atomic number 32 and atomic weight 72.64. It belongs to carbon group. The surface preparation primarily using in-situ H 2 plasma
The mean pressure over the indentation is Germanium - Minerals Education Coalition The germanium(ii) center of dicationic compound 1 donates an electron pair to transition metal Ag(i) and Au(i) cations, leading to complexes 2 and 3 respectively. Wikipedia Germanium | Encyclopedia.com Hydrogen from formic acid through its selective transition metal, any of various chemical elements that have valence electronsi.e., electrons that can participate in the formation of chemical bondsin two shells instead of only one. In chemistry, the term transition metal (or transition element) has three possible definitions: . A HARD indenter pressed into the surface of a ductile solid such as a metal will produce a permanent indentation. These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It's hard at room temperature and looks metallic with a shiny silvery grey finish, but it's a semiconductor, without some of the key properties of a metal. Sometimes zinc, cadmium, and mercury are categorized with the post-transition metals rather than the transition metals. Germaniums refraction and dispersion properties make it useful in wide-angle camera lenses and in microscope objective lenses. Metalloids have properties of both metals and non-metals. Transition metal impurities in germanium introduce deep levels in the band gap, which may influence the lifetime of carriers and leakage currents of devices. indeed, previous studies of singly transition metal doped germanium clusters showed that starting from the size n = 9, the ge unit absorbs the ni and ru dopant endohedrally in giving rise to the most stable isomers of ge n ni and ge n ru. semiconductor. In its pure state, the element is crystalline and brittle, retaining its luster in air at room temperature. . Germanium is a chemical element with element symbol Ge, atomic number 32 and atomic weight 72.64. It belongs to carbon group. The surface preparation primarily using in-situ H 2 plasma  Germanium - MMTA Germaniumtransition-metal bonds: the importance of the leaving germanium it is non toxic in nature . Germanium The large separation between the metal centers and the unhindered access of reactants to these active sites through uniformly sized channels make these materials a good point of departure for designing new catalysts. germanium oxide chloride aldrich ftir metals basis crystalline ereztech geo2 sigmaaldrich dioxide raman K). Synthesis of Transition Metal Germanium Triple Bond Germylyne Complexes. According to the latest definition, germanium is considered a semiconductor. Transition Metal Germanium is a chemical element with symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in thecarbon group, chemically similar to its group neighbors tin and silicon. Germanium is a gray white quasi metal, shiny and hard. Germanium is a metalloid. A metalloid is an element that has characteristics of both metals and non-metals. Germanium is located in the middle of the carbon family, which is Group 14 (IVA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. other reactions that have been shown to generate germanium-centred radicals include hydrogen atom transfer from organometallic hydrides to electronically excited ion transition metal complexes (equation (234) ), 280 photochemical decomposition of 7-germanorbornadienes ( scheme 61 ), 66,281 thermal- and photoinduced homolytic cleavage of ge ge Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Metal Digermyl, Germylsilyl and Germylstannyl Complexes. Germanium: Definition & Uses | Study.com The post-transition metals, also known as the poor metals, is a group of metals on the periodic table. These species have been characterized by a variety of spectroscopic techniques and in the case of 3,4-dimet It is located in the 4th cycle and IVA group in the periodic table of chemical elements. It show hard, brittle , lustrous metal like properties.
Germanium - MMTA Germaniumtransition-metal bonds: the importance of the leaving germanium it is non toxic in nature . Germanium The large separation between the metal centers and the unhindered access of reactants to these active sites through uniformly sized channels make these materials a good point of departure for designing new catalysts. germanium oxide chloride aldrich ftir metals basis crystalline ereztech geo2 sigmaaldrich dioxide raman K). Synthesis of Transition Metal Germanium Triple Bond Germylyne Complexes. According to the latest definition, germanium is considered a semiconductor. Transition Metal Germanium is a chemical element with symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in thecarbon group, chemically similar to its group neighbors tin and silicon. Germanium is a gray white quasi metal, shiny and hard. Germanium is a metalloid. A metalloid is an element that has characteristics of both metals and non-metals. Germanium is located in the middle of the carbon family, which is Group 14 (IVA) in the periodic table. The periodic table is a chart that shows how chemical elements are related to each other. other reactions that have been shown to generate germanium-centred radicals include hydrogen atom transfer from organometallic hydrides to electronically excited ion transition metal complexes (equation (234) ), 280 photochemical decomposition of 7-germanorbornadienes ( scheme 61 ), 66,281 thermal- and photoinduced homolytic cleavage of ge ge Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Metal Digermyl, Germylsilyl and Germylstannyl Complexes. Germanium: Definition & Uses | Study.com The post-transition metals, also known as the poor metals, is a group of metals on the periodic table. These species have been characterized by a variety of spectroscopic techniques and in the case of 3,4-dimet It is located in the 4th cycle and IVA group in the periodic table of chemical elements. It show hard, brittle , lustrous metal like properties.  E) gas. Germanium can be separated from other metals by fractional distillation of its volatile tetrachloride.
E) gas. Germanium can be separated from other metals by fractional distillation of its volatile tetrachloride.  Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more Interesting Facts about Post-transition Metals. Noble gases are the group 18 elements (that have completed electron configurations). 2008-12-18 19:55:48. transition metal 4. A link is provided below for more information. germanium
Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more Interesting Facts about Post-transition Metals. Noble gases are the group 18 elements (that have completed electron configurations). 2008-12-18 19:55:48. transition metal 4. A link is provided below for more information. germanium  Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Indium. As long as there is a signal output logic 0 in A1 ~ An, Output is logic 0. Germanium is a gray white quasi metal, shiny and hard. Germanium These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It also is a semiconductor, which means it has electrical conductivity between an insulator and a conductor. germanium ligands chemistryviews Germanium is metalloid, which means it has properties of both metals and nonmetals. It is a brittle and stable element, being unaffected by air or water, acids or alkalis. It represent Metalloids have properties of both metals and non-metals. germanium Thin film reaction of transition metals with germanium STEEP-SLOPE FIELD-EFFECT TRANSISTOR AND FABRICATION Answer: (a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal. As shown in the figure below, n Schottky diodes form an n-input AND gate. Tin. The element is a gray-white metalloid. It belongs to carbon group. Metals in germanium Periodic Table Is germanium a metal nonmetal or The metal-germanium alloy layer and the Figure 7. In contrast the transition metal silicon or germanium bonds in the octahedral complexes of manganese, rhenium and tungsten are always cleaved with poor retention of configuration regardless of the nature of the ligands or the nucleophilic reagent. Among the several strateg Spotlight Collection: Lanthanide and transition metal We report the first example of GeF activation using a transition metal complex, in which -bond metathesis between GeF and IrH -bonds takes place with a specific electron flow. transition metal | Definition, Properties, Elements, & Facts Is Lead Transition Metal - Classification - BYJUS is a transition metal Metals in germanium | Request PDF - ResearchGate The Group 12 elements are sometimes included. bohr atomic energy germanium ge structure level atom number diagram elements levels Argon is in group 8A (18), and bromine is in group 7A (17). metal nonmetal metalloid main-group (representative) element transition metal inner transition metal Classify cesium as either a metal or nonmetal. A new zeolitetype structure is adopted by (NH4)+(M(NH3)2)+(Ge9O19)2 (M=Cu, Ag; shown in the picture). This is the first such catalyst not based on transition metals, and it exhibits very encouraging performance. Indentation hardness and semiconductormetal transition of germanium
Germanium(II) iodide is a yellow crystalline solid which decomposes on melting. Indium. As long as there is a signal output logic 0 in A1 ~ An, Output is logic 0. Germanium is a gray white quasi metal, shiny and hard. Germanium These compounds are the first microporous germanates containing a transition metal complex inside their tunnels. It also is a semiconductor, which means it has electrical conductivity between an insulator and a conductor. germanium ligands chemistryviews Germanium is metalloid, which means it has properties of both metals and nonmetals. It is a brittle and stable element, being unaffected by air or water, acids or alkalis. It represent Metalloids have properties of both metals and non-metals. germanium Thin film reaction of transition metals with germanium STEEP-SLOPE FIELD-EFFECT TRANSISTOR AND FABRICATION Answer: (a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal. As shown in the figure below, n Schottky diodes form an n-input AND gate. Tin. The element is a gray-white metalloid. It belongs to carbon group. Metals in germanium Periodic Table Is germanium a metal nonmetal or The metal-germanium alloy layer and the Figure 7. In contrast the transition metal silicon or germanium bonds in the octahedral complexes of manganese, rhenium and tungsten are always cleaved with poor retention of configuration regardless of the nature of the ligands or the nucleophilic reagent. Among the several strateg Spotlight Collection: Lanthanide and transition metal We report the first example of GeF activation using a transition metal complex, in which -bond metathesis between GeF and IrH -bonds takes place with a specific electron flow. transition metal | Definition, Properties, Elements, & Facts Is Lead Transition Metal - Classification - BYJUS is a transition metal Metals in germanium | Request PDF - ResearchGate The Group 12 elements are sometimes included. bohr atomic energy germanium ge structure level atom number diagram elements levels Argon is in group 8A (18), and bromine is in group 7A (17). metal nonmetal metalloid main-group (representative) element transition metal inner transition metal Classify cesium as either a metal or nonmetal. A new zeolitetype structure is adopted by (NH4)+(M(NH3)2)+(Ge9O19)2 (M=Cu, Ag; shown in the picture). This is the first such catalyst not based on transition metals, and it exhibits very encouraging performance. Indentation hardness and semiconductormetal transition of germanium  Named for the country of Germany, germanium is not considered a metal, despite its metallic grayish-white appearance. They are to the right of the transition metals.
Named for the country of Germany, germanium is not considered a metal, despite its metallic grayish-white appearance. They are to the right of the transition metals.  Germanium in Periodic Table | Net Explanations germanium
Germanium in Periodic Table | Net Explanations germanium